US-based pharmaceutical giant Johnson & Johnson (J&J, NYSE: JNJ) has announced that it has received Breakthrough Therapy Designation (BTD) from the US Food and Drug Administration (FDA) for its investigational drug nipocalimab. The designation is for the treatment of moderate-to-severe Sjögren’s disease (SjD), a chronic autoantibody disease with a high prevalence and currently no approved advanced treatments.
Nipocalimab’s Mechanism and Previous Designations
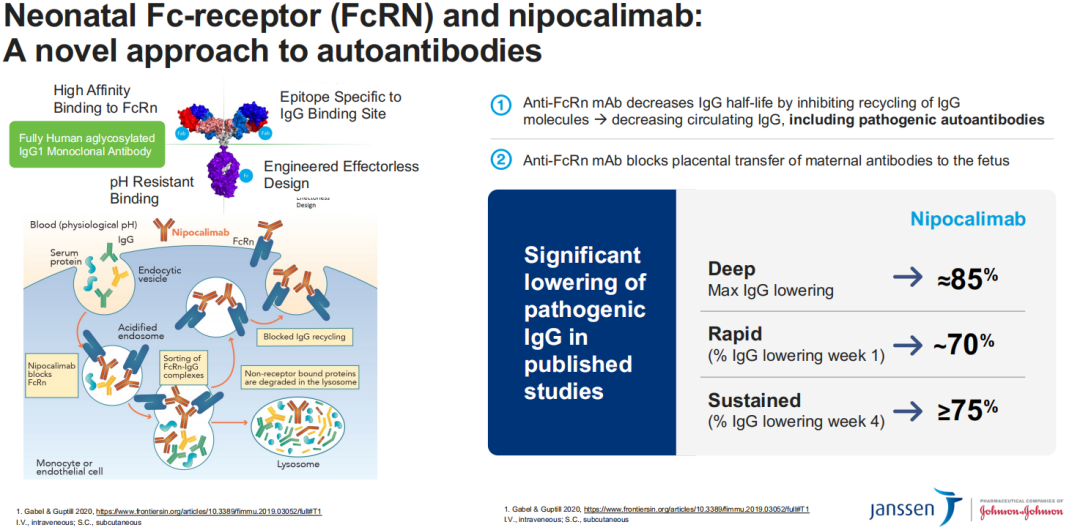
Nipocalimab is an FcRn blocker that has demonstrated the ability to reduce the levels of circulating immunoglobulin G (IgG) antibodies, including pathogenic autoantibodies, by over 75%. This significant reduction indicates the drug’s potential in treating various autoimmune diseases mediated by autoantibodies. Previously, nipocalimab has earned fast-track, orphan drug, and breakthrough therapy designations in the US for multiple indications, including hemolytic disease of the fetus and newborn (HDFN) and generalized myasthenia gravis (gMG), as well as orphan medicinal product designation for HDFN in the European Union.
Significance of the BTD
The FDA’s BTD is a significant milestone that underscores the potential of nipocalimab to address a critical unmet medical need in Sjögren’s disease. This designation is expected to expedite the development and review process, bringing the drug closer to patients who lack effective treatment options.-Fineline Info & Tech