Shanghai-based LaNova Medicines Ltd, a developer of cancer therapies, has announced another licensing deal, signing an agreement with compatriot firm Sino Biopharmaceutical Ltd (HKG: 1177) for the development and commercialization of LM-108 and other potential bispecific antibodies (BsAbs) and antibody drug conjugates (ADCs) in mainland China. The specifics of the partnership agreement have not been fully disclosed. This deal follows closely on the heels of a significant agreement with US pharmaceutical giant Merck, Sharp & Dohme Inc. (MSD; NYSE: MRK), which acquired LaNova’s PD-1/VEGF BsAb LM-299 in a deal valued at USD 3.3 billion late last week.
Sino Biopharmaceutical’s Equity Investment in LaNova
In addition to the licensing agreement, Sino Biopharmaceutical has also entered into an equity investment with LaNova, acquiring a 4.91% stake in the company for RMB 142 million (USD 19.61 million). This investment comes on the back of Sino Bio’s support for LaNova’s RMB 300 million (USD 42.1 million) Series C1 financing round concluded late last month.
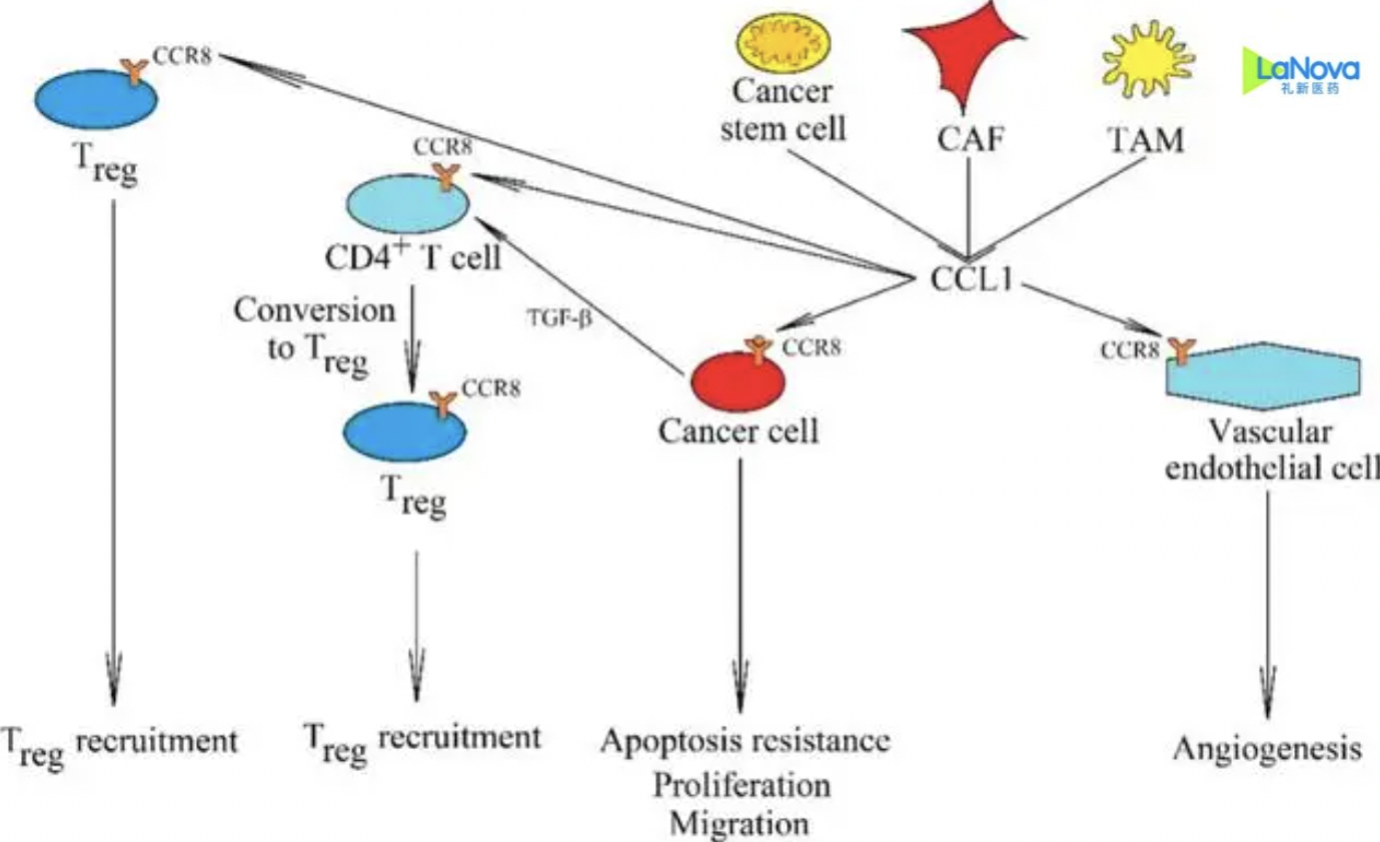
LM-108: LaNova’s Monoclonal Antibody in Phase II Clinical Study
LM-108, an in-house developed monoclonal antibody (mAb) targeting CCR8, is currently the subject of a Phase II clinical study. This development highlights LaNova Medicines’ commitment to advancing innovative immunotherapies for the treatment of cancer.-Fineline Info & Tech