Shanghai Escugen, a China-based biopharmaceutical company, has announced receiving the green light from the National Medical Products Administration (NMPA) to proceed with a second Phase III study for its antibody drug conjugate (ADC), ESG401. The study will focus on unresectable locally advanced, recurrent, or metastatic PD-L1 negative triple negative breast cancer (TNBC) patients who have not received systematic treatment in the past. The first subject enrollment is anticipated to commence at the beginning of January 2025.
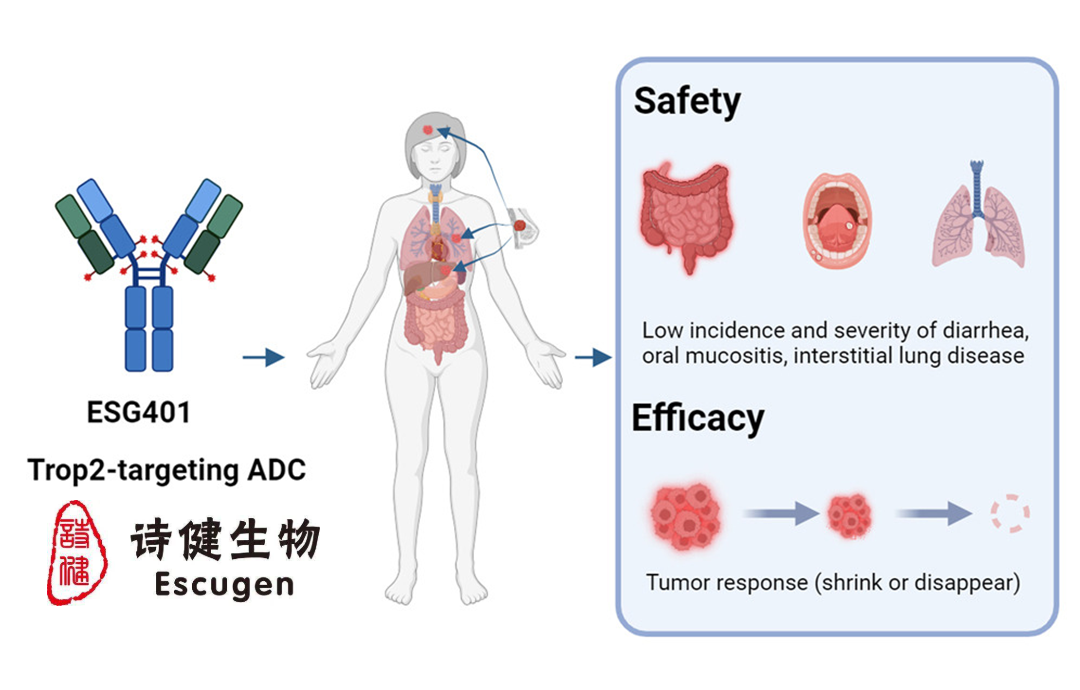
ESG401: An Antibody Drug Conjugate Targeting Trop-2
ESG401, which targets Trop-2, has demonstrated superiority over similar products in existing clinical results. The drug has shown a higher tolerated dose, lower incidence, and milder degree of off-target and on-target toxicity, providing significant safety advantages. These positive attributes position ESG401 as a promising candidate for the treatment of TNBC, a particularly aggressive form of breast cancer.
Implications of the Second Phase III Trial
The approval for a second Phase III trial by the NMPA is a significant milestone for Shanghai Escugen, as it seeks to expand the clinical evidence supporting the safety and efficacy of ESG401. This additional study will further evaluate the drug’s potential to improve outcomes for patients with TNBC, who often face limited treatment options.-Fineline Info & Tech