China-based cancer immunotherapy specialist Novatim Immune Therapeutics (Zhejiang) Co., Ltd. has announced receiving clinical clearance from the US Food and Drug Administration (FDA) for its innovative drug candidate, Y-0301, a bispecific nanobody targeting MET and EGFR pathways.
Y-0301: A Pioneering Bispecific Nanobody
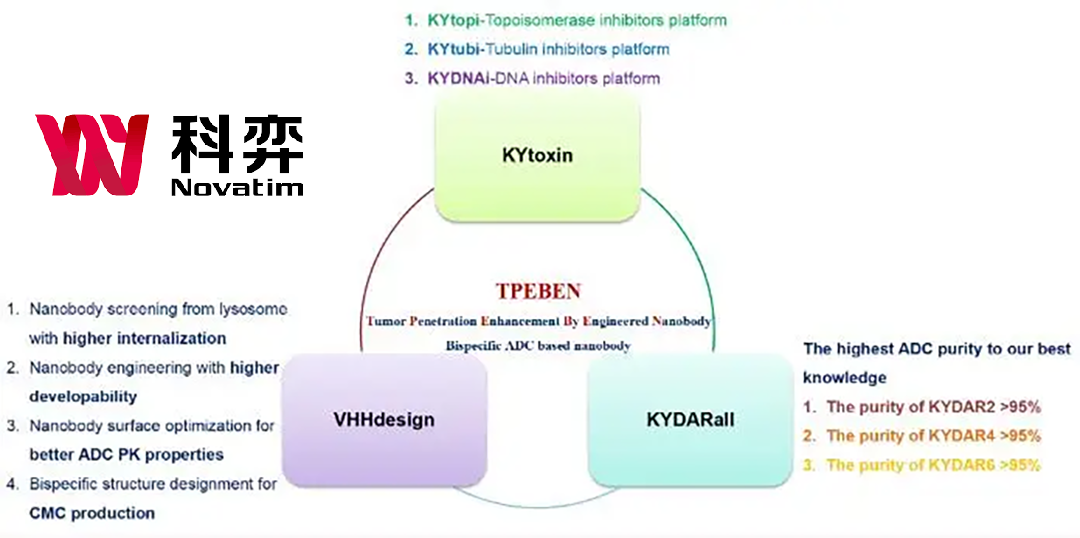
Y-0301, the world’s first bispecific nanobody developed through Novatim’s proprietary nanobody platform TPEBEN, has been engineered with a lower EGFR affinity (161-fold) and EGFR arm truncation. These unique design features allow Y-0301 to offer far superior safety compared to similar products in the market.
Preclinical Studies Highlight Y-0301’s Efficacy and Safety
In preclinical studies, Y-0301 demonstrated more effective tumor tissue penetration and higher anti-tumor activity compared to traditional bispecific antibodies. Its superior safety profile positions Y-0301 as a promising candidate in the field of cancer immunotherapy, potentially offering a new treatment option for patients with MET and EGFR-driven cancers.-Fineline Info & Tech