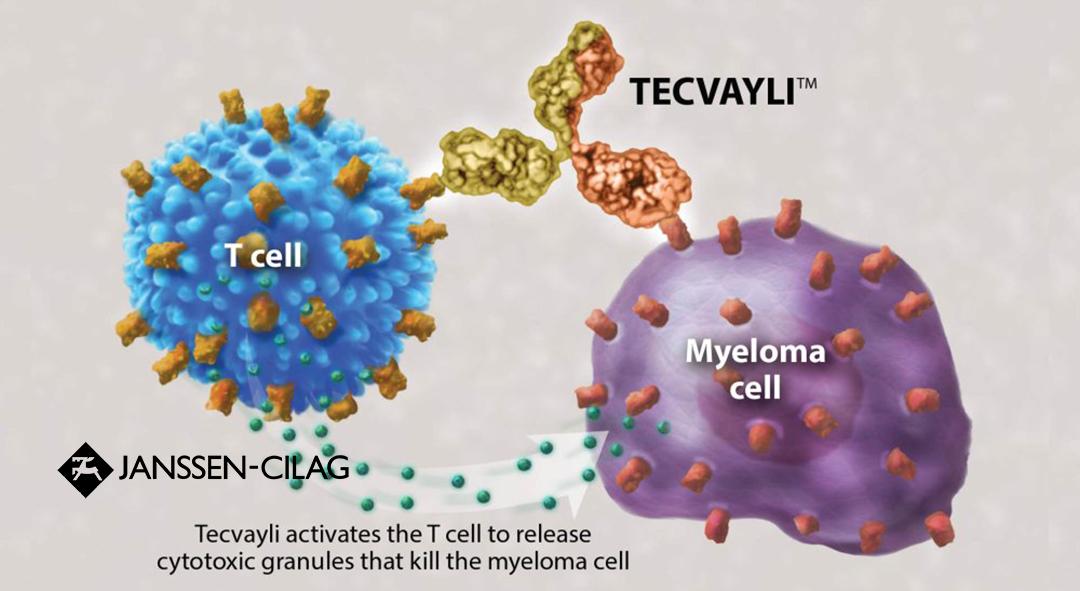
Janssen-Cilag International NV, a subsidiary of US healthcare giant Johnson & Johnson (J&J; NYSE: JNJ), announced the presentation of results from the MajesTEC-5 and MajesTEC-4 studies for its bispecific antibody (BsAb) Tecvayli (teclistamab) at the 66th American Society of Hematology (ASH) annual meeting. The studies focused on patients with newly diagnosed multiple myeloma (NDMM) in both induction and maintenance settings.
Tecvayli’s Approval and Study Design
Tecvayli, which targets CD3 and BCMA, received marketing approval in China in June this year for use in relapsed or refractory multiple myeloma (r/rMM) after three or more prior lines of therapy. The MajesTEC-5 study involved 49 NDMM patients eligible for transplantation who were treated with teclistamab in combination with Darzalex (daratumumab) subcutaneous (SC) formulation, lenalidomide, and dexamethasone (Tec-DRd) or daratumumab SC, bortezomib, lenalidomide, and dexamethasone (Tec-DVRd) as induction therapy. All patients evaluated for minimal residual disease (MRD) negativity after cycle 3 of induction therapy achieved MRD negativity (10-5) and maintained this through cycle 6.
MajesTEC-4 Study Results and Future Analysis
The MajesTEC-4 study aims to assess teclistamab in combination with lenalidomide and teclistamab alone versus lenalidomide alone in participants with NDMM as maintenance therapy following autologous stem cell transplantation (ASCT). Initial safety run-in results showed low rates of non-haematologic Grade 3/4 treatment-emergent adverse events (TEAEs) and a 5.3 percent discontinuation rate due to all TEAEs. Further analysis of combination therapies will be conducted in the Phase III MajesTEC-7 study, which is currently enrolling subjects.-Fineline Info & Tech