Beijing-based gene therapy specialist InnoVec Biotherapeutics Inc. has announced receiving Investigational New Drug (IND) approval from the US Food and Drug Administration (FDA) for its IVB102. This development follows the completion of enrollment in the investigator-initiated trial (ITT) at Peking Union Medical College Hospital. The FDA clearance was granted based on the company’s vitreous drug delivery carrier platform and outstanding IIT data.
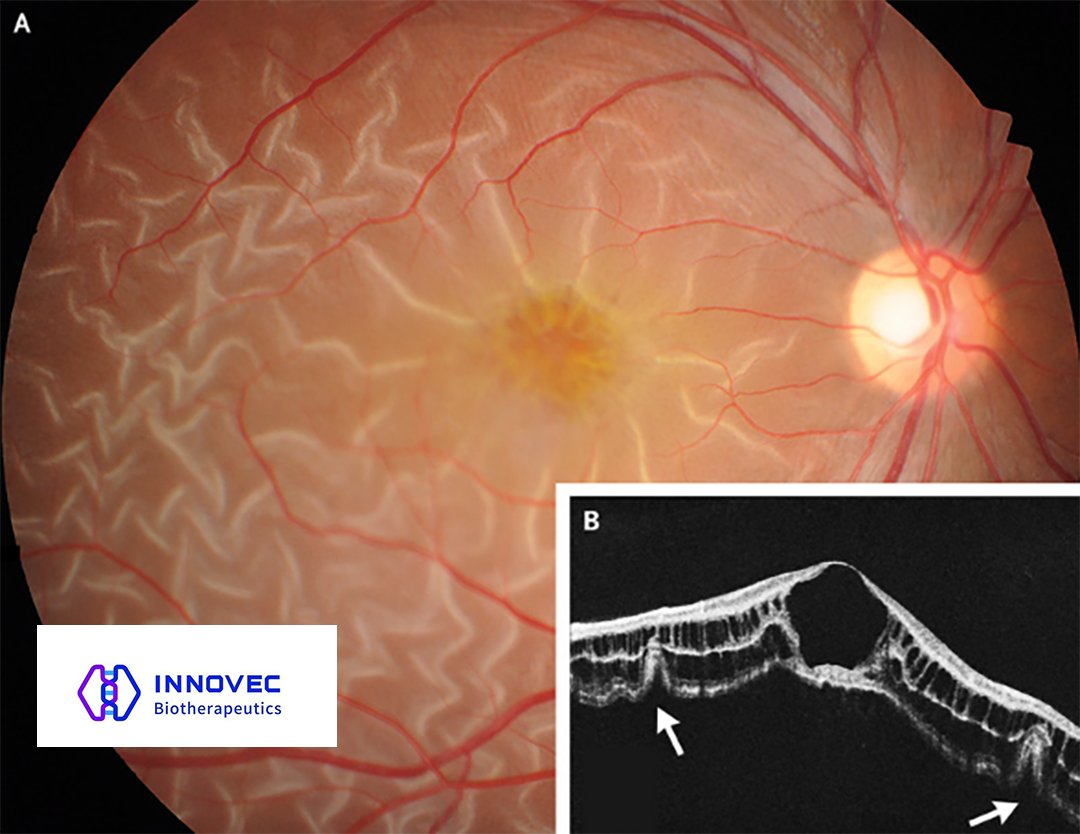
IVB102: A Promising Therapy for X-Linked Retinoschisis (XLRS)
IVB102 is an intravitreal injection therapy designed for the treatment of X-linked retinoschisis (XLRS), a genetic disorder affecting the retina. Pre-clinical studies have demonstrated IVB102’s potential for best-in-class therapeutic effects, positioning it as a promising candidate for further clinical development.
The Significance of FDA IND Approval
The FDA’s IND approval is a significant milestone for InnoVec Biotherapeutics, as it allows the company to proceed with clinical trials in the US, expanding the global reach of its gene therapy research. This approval also reflects the potential impact of IVB102 on the treatment landscape for XLRS patients, offering hope for a new and effective therapy.-Fineline Info & Tech