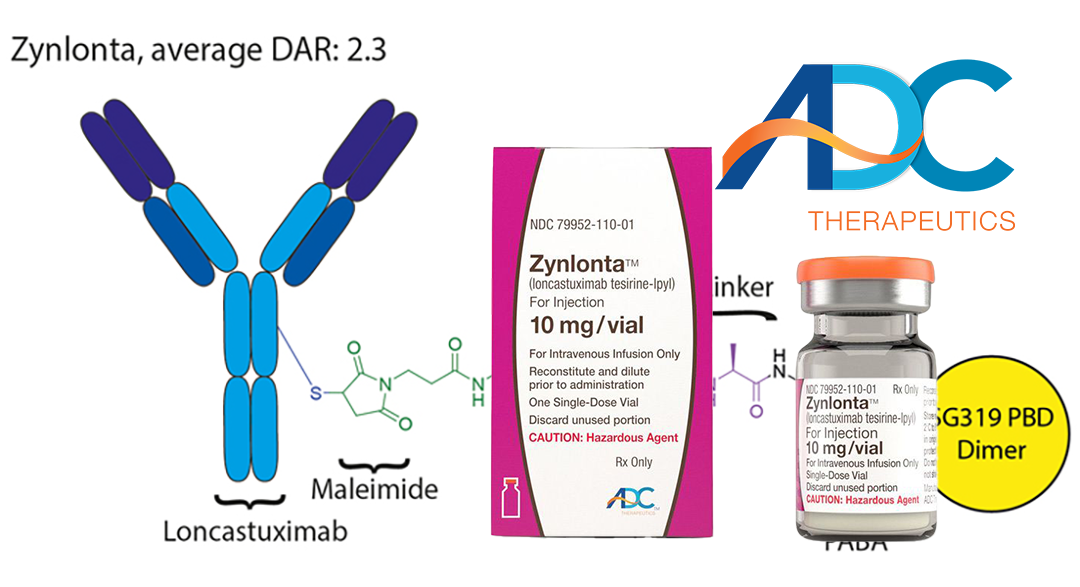
The website of China’s National Medical Products Administration (NMPA) has indicated that the marketing filing for Zynlonta (loncastuximab tesirine), a collaboration between China’s Overland Pharmaceuticals and Swiss firm ADC Therapeutics SA (NYSE: ADCT), has been approved. This approval allows the CD19-targeted antibody-drug conjugate (ADC) to be used for treating adult patients with relapsed or refractory large B-cell lymphoma (r/r DLBCL) after second-line or multi-line systemic therapy.
Phase II Study Results and Global Consistency
The results of the Phase II regulatory OL-ADCT-402-001 study demonstrated that Zynlonta had good efficacy and safety in Chinese patients with r/r DLBCL. The primary endpoint of overall response rate (ORR) was met, with benefit status on par with the global pivotal ADCT-402-201 study. This consistency in efficacy and safety across studies further validates Zynlonta’s potential as a treatment option for patients with r/r DLBCL.
Fast-Track Approval and Global Leadership
Loncastuximab tesirine was fast-tracked in the US in April 2021 for use in adults with r/r DLBCL who have received at least two lines of systemic therapy. It remains the first and only CD19-targeted ADC globally. Overland ADCT BioPharma (CY) Ltd, a joint venture (JV) established between Overland Pharma and ADC Therapeutics in December 2020, holds the rights to the drug in Greater China and Singapore.-Fineline Info & Tech