China-based IMEIK Technology Development Co., Ltd (SHE: 300896) has announced receiving approval from the National Medical Products Administration (NMPA) to conduct a clinical study for its recombinant human hyaluronidase for injection. The study aims to explore the use of the drug to promote the diffusion of subcutaneous infusion as an alternative to intravenous infusion.
Hyaluronidase’s Broad Medical Applications
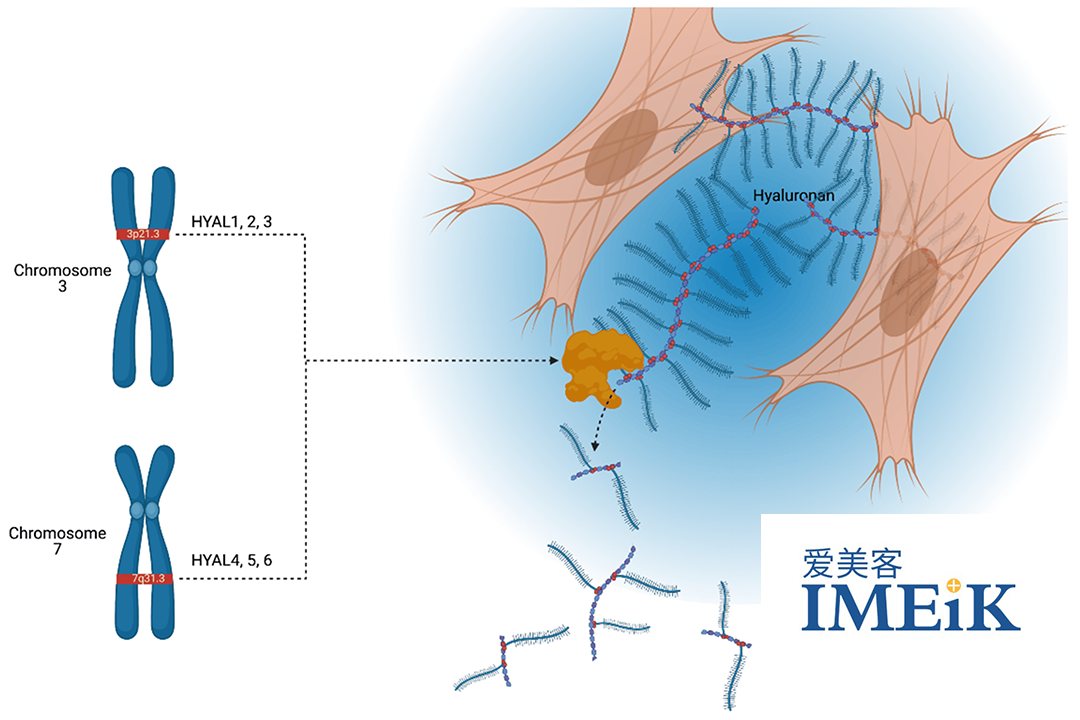
Hyaluronidase is a versatile enzyme widely used across various medical fields, including medical aesthetics, ophthalmic surgery, drug delivery, and assisted reproduction. The enzyme’s ability to decompose hyaluronic acid and assist drug diffusion, as well as its role in preventing postoperative tissue adhesion, makes it an important tool in surgical and therapeutic procedures.
Historical Context and Market Landscape
Biochemically extracted animal-derived hyaluronidase first entered the market in the United States in 1948, followed by products such as Vitrase, Amphadase, and other injectable forms of hyaluronidase. Hylenex, which received marketing approval in the US in 2005, stands as the world’s only recombinant human hyaluronidase. The development of IMEIK Technology’s recombinant human hyaluronidase injection represents a significant advancement in the field, potentially offering a more effective and safer alternative for patients.-Fineline Info & Tech