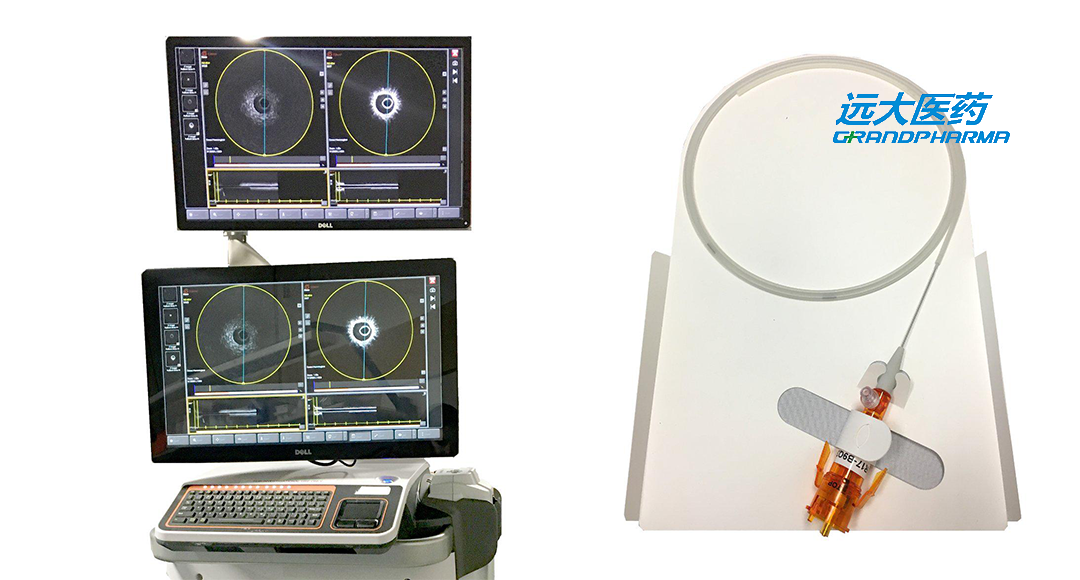
China-based Grand Pharmaceutical Group Co., Ltd. (HKG: 0512) has announced that it has received marketing approval from China’s National Medical Products Administration (NMPA) for its innovative intravascular dual-mode imaging system, NOVASYNC HYBRID SYSTM.
Milestone in Vascular Intervention Technology
NOVASYNC, a domestic substitute for Grand Pharma’s imported NOVASIGHT HYBRID SYSTEM, signifies a significant advancement for the company in the field of vascular intervention for precise diagnosis and treatment of cardiovascular and cerebrovascular diseases. This new product integrates two advanced imaging technologies: intravascular ultrasound (IVUS) and optical coherence tomography (OCT). The system’s capability to display intravascular ultrasound and optical images simultaneously, in the same direction, and from a coaxial and same positional view, underscores its potential for broad application in coronary angiography and endovascular intervention surgery.
Enhancing Diagnostic and Treatment Capabilities
The approval of NOVASYNC HYBRID SYSTM is a testament to Grand Pharmaceutical Group’s commitment to enhancing diagnostic and treatment capabilities in the vascular intervention space. The system’s dual-mode imaging provides clinicians with a comprehensive view of the vascular structure, which is crucial for making informed decisions during complex procedures. This approval not only expands the company’s product portfolio but also contributes to the domestic medical device industry’s growth, offering an alternative to imported systems and potentially improving patient outcomes through advanced imaging technologies.-Fineline Info & Tech