Lepu Scientech Medical Technology (Shanghai) Co., Ltd (HKG: 2291), a leading China-based medical technology company, has announced that it has received a medical device license from the National Medical Products Administration (NMPA) for its innovative ScienCcrown transcatheter implantable aortic valve system. This development marks a significant milestone in the advancement of cardiac care in China.
Innovative Design Addresses TAVR Surgery Challenges
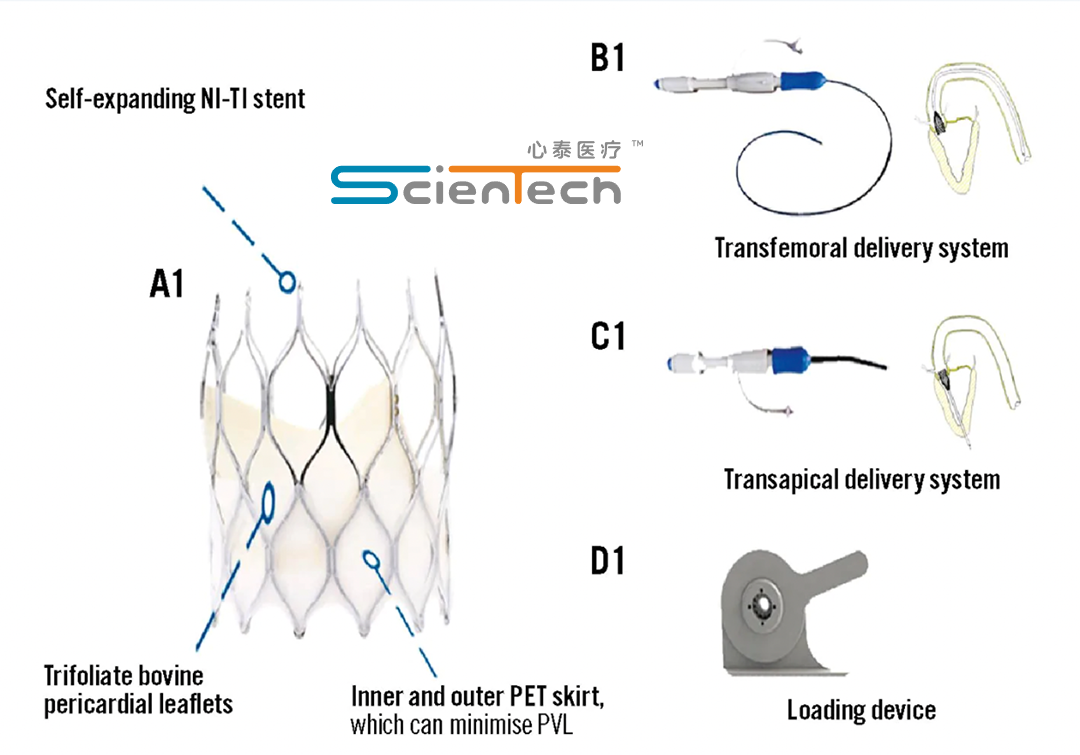
The ScienCcrown system is designed with a focus on clinical practice, effectively addressing common challenges in transcatheter aortic valve replacement (TAVR) surgery. These include poor valve dilation, inaccurate positioning, the risk of displacement, and coronary artery occlusion. The system’s use of anti-calcification bovine pericardial valve leaflets ensures higher durability without impeding the opening of the coronary artery or affecting re-intervention surgery, making it a safe and effective choice for patients.
Advantages of the ScienCcrown System
The ScienCcrown system’s design combines compliance and support, which is instrumental in reducing long-term complications. It offers stable full recycling and repeated positioning, ensuring precise valve placement. The system’s ease of operation and safety make it a preferred option for interventional treatment. Moreover, the dual approach capability, through both the femoral artery and the apex of the heart, provides additional options for interventional treatment, catering to diverse clinical needs.-Fineline Info & Tech