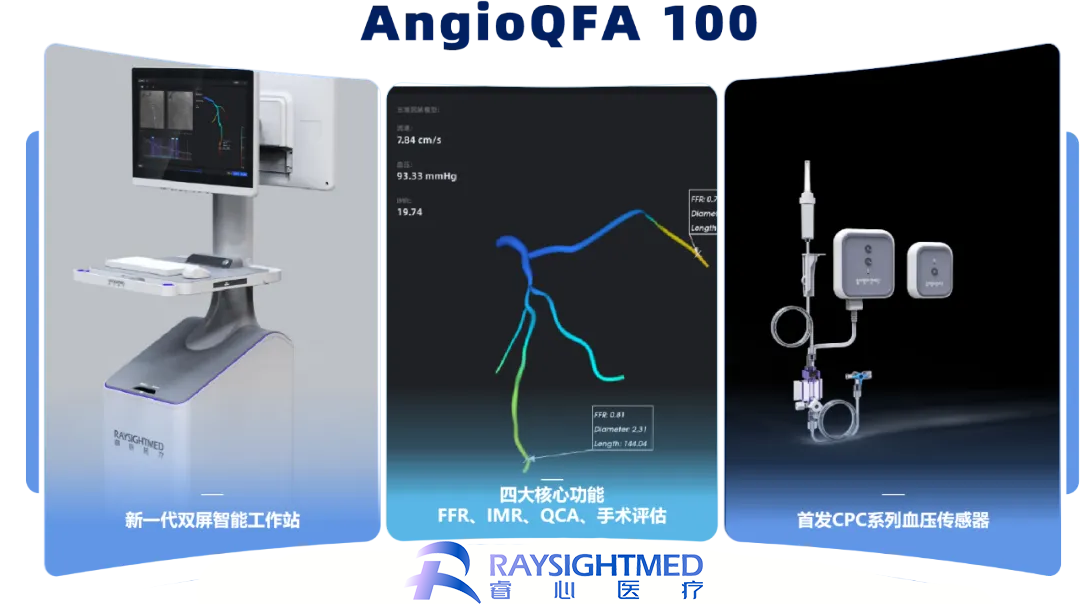
China-based RaysightMed Co., Ltd has announced that it has received marketing approval from the National Medical Products Administration (NMPA) for its next-generation FFR imaging product, AngioQFA 100, a revolutionary coronary artery function measurement system.
Advanced Fusion Technology for Comprehensive Assessment
The AngioQFA 100 system employs a sophisticated fusion of artificial intelligence (AI) and fluid mechanics to deliver precise, comprehensive, flexible, and efficient assessments of coronary blood supply. This advanced technology allows for a single analysis that covers multiple blood vessels, simultaneously obtaining scores of coronary fractional flow reserve (FFR) and the index of microcirculation resistance (IMR). This capability effectively supports the entire process of interventional percutaneous coronary intervention (PCI) surgery.
Enhancing Interventional Cardiology Procedures
The approval of AngioQFA 100 by the NMPA marks a significant advancement in the field of interventional cardiology. The system’s ability to provide detailed assessments of coronary blood flow and microcirculation resistance in a single procedure streamlines surgical planning and execution. This innovation is set to improve patient outcomes and enhance the efficiency of cardiovascular procedures in China.-Fineline Info & Tech