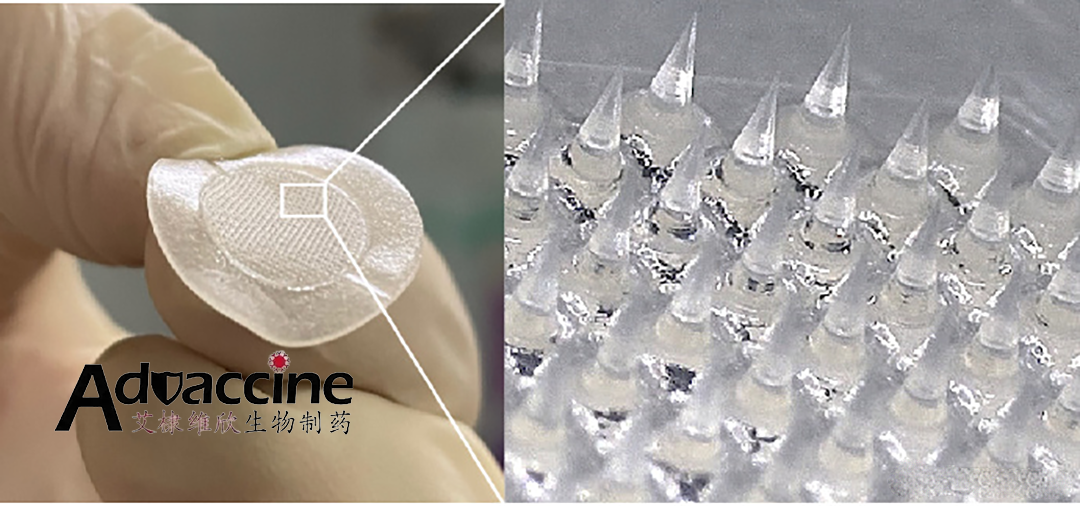
China-based vaccine developer Advaccine Biotechnology has announced receiving registration approval from the US Food and Drug Administration (FDA) for its soluble microneedle drug, MICROEPAD.
MICROEPAD: A Revolutionary Approach to Skin Treatments
MICROEPAD, or Dissolvable Microneedle Blemish Patches, is an over-the-counter (OTC) medication designed to treat acne, acne scars, and blackheads, promoting skin healing. This innovative product prepares drug ingredients into soluble microneedle formulations, which are applied locally to the skin. The microneedles then dissolve and release the drug ingredients through the stratum corneum, offering a new method for topical drug delivery.
The Impact of MICROEPAD on Skin Care
The approval of MICROEPAD by the FDA marks a significant advancement in the field of dermatological treatments. This soluble microneedle technology has the potential to revolutionize how acne and related skin conditions are managed, providing patients with an effective and convenient treatment option. The local application and dissolution of the microneedles allow for targeted drug delivery, which can lead to improved healing and reduced side effects commonly associated with traditional topical medications.-Fineline Info & Tech