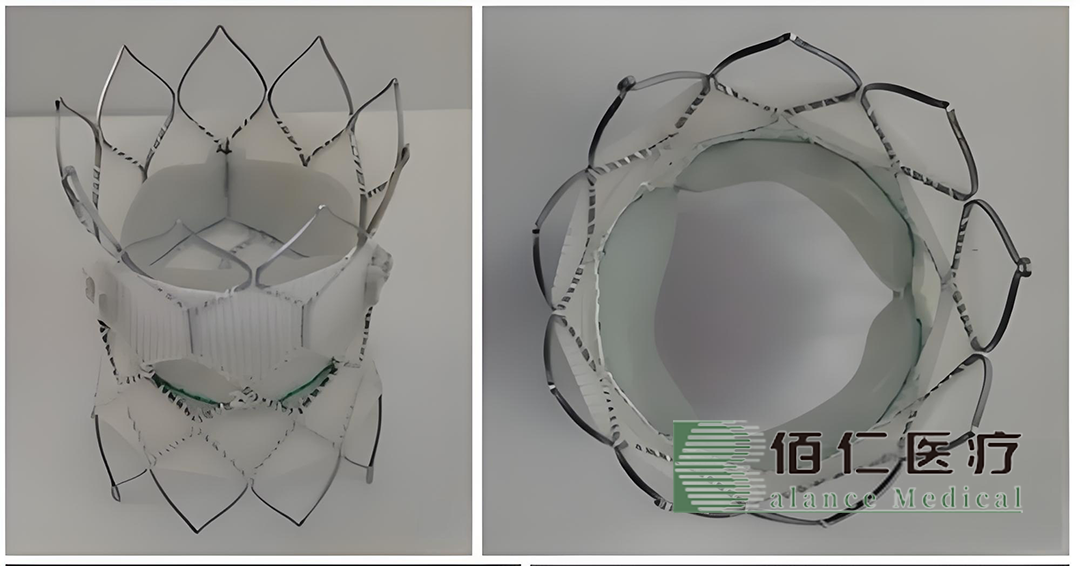
China’s Beijing Balance Medical Technology Co., Ltd. (SHA: 688198) has announced that it has received fast-track designation from the Center for Medical Device Evaluation, NMPA, for its in-house developed all-comer interventional pulmonary valve and delivery system. This marks the third interventional valve product from Balance Medtech to receive fast-track status in China.
Targeting Complex Congenital Heart Disease Patients
The product is specifically designed for patients with complex congenital heart disease who require transcatheter artificial biological pulmonary valve replacement after surgical right ventricular outflow tract repair and reconstruction. This innovative solution aims to address the unique needs of this patient population.
Unique Design and Preoperative Imaging Analysis
A key feature of the company’s product design is the use of preoperative imaging analysis to determine the entry point through the chest. The main pulmonary artery is first reshaped through the chest, and then the product is intervened through a catheter. This approach ensures that the product can meet the needs of any patient with main pulmonary artery malformation who is eligible for transcatheter intervention for pulmonary valve treatment.-Fineline Info & Tech