Zai Lab Limited (NASDAQ: ZLAB, HKG: 9688) announced that a Biological License Application (BLA) for TIVDAK (tisotumab vedotin-tftv), an antibody-drug conjugate (ADC) originated by Seagen Inc., has been accepted for review by China’s National Medical Products Administration (NMPA). This marks a significant step forward in making this innovative therapy available to patients in China.
Drug Profile and Mechanism
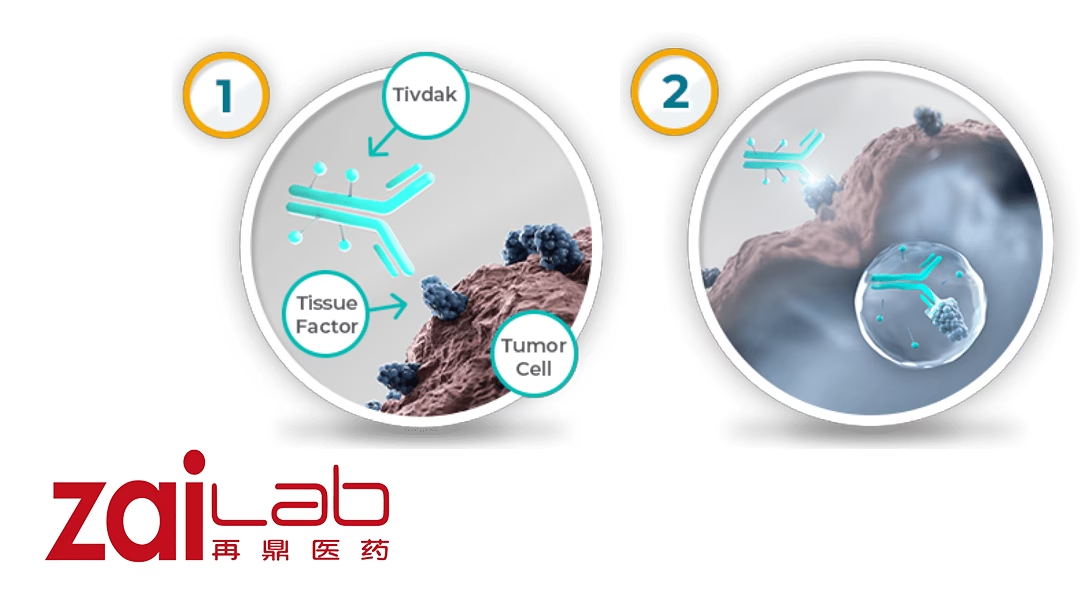
TIVDAK is an ADC composed of Genmab’s human monoclonal antibody directed to tissue factor (TF) and Seagen’s ADC technology. It utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E (MMAE) to the antibody. This mechanism allows for targeted delivery of chemotherapy to cancer cells, potentially improving efficacy and reducing side effects.
Clinical Data and Approval Timeline
The BLA submission is supported by robust clinical data demonstrating TIVDAK’s efficacy and safety in patients with recurrent or metastatic cervical cancer who have experienced disease progression after systemic therapy. The drug received full FDA approval in April 2024 for similar indications, highlighting its potential to address significant unmet medical needs.-Fineline Info & Tech