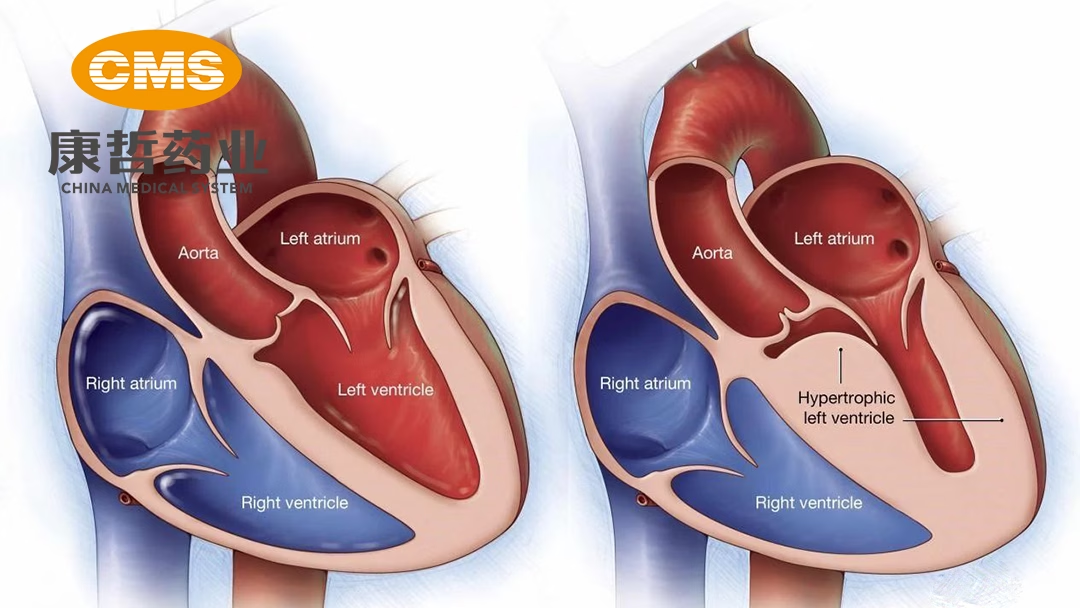
China Medical System Holdings (CMS; HKG: 0867) announced receiving approval from the National Medical Products Administration (NMPA) to conduct a clinical study evaluating the safety, tolerability, pharmacokinetics, and pharmacological characteristics of its investigational drug CMS-D003. The study will focus on healthy adults and those with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) in China.
Drug Mechanism and Potential
CMS-D003 is a small molecule cardiac myosin inhibitor designed to target the inhibition of cardiac myosin triphosphate adenosine (ATP) enzyme. By suppressing myosin-actin interaction, it reduces muscle filament sliding and inhibits excessive myocardial contraction. This mechanism aims to improve diastolic dysfunction of the heart and alleviate clinical symptoms in patients with oHCM.
Clinical Significance
The drug’s short half-life, low risk of drug-drug interactions, and favorable safety profile position it as a potential treatment for heart failure with preserved ejection fraction. The clinical study represents a crucial step in advancing CMS’s pipeline and addressing significant unmet medical needs in cardiovascular diseases.-Fineline Info & Tech