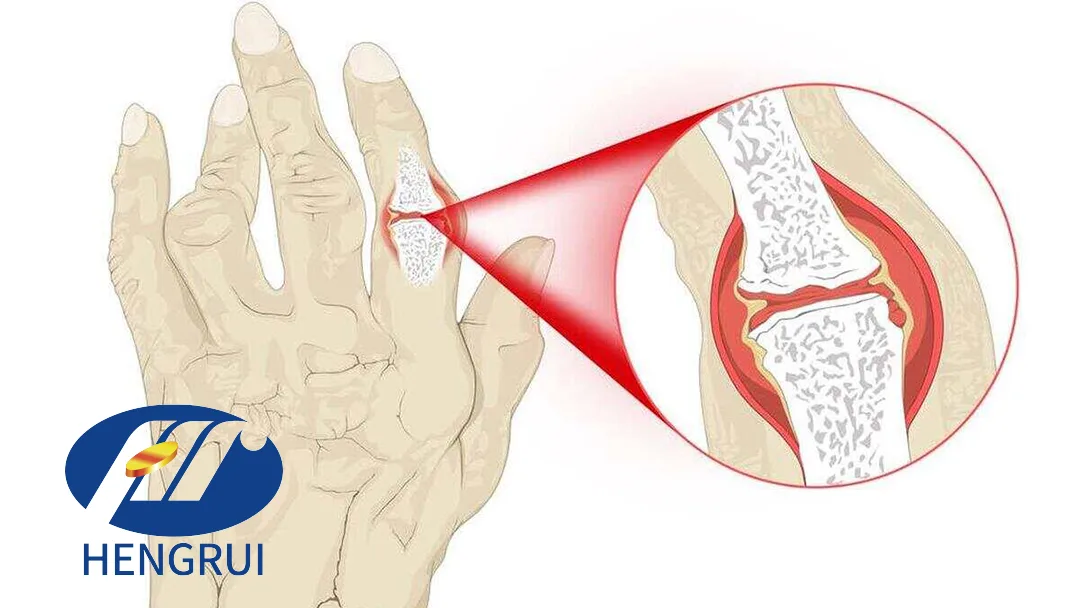
Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) announced that it has received additional indication approval from the National Medical Products Administration (NMPA) for its JAK1 inhibitor ivarmacitinib. The drug, recently approved for active ankylosing spondylitis (AS) in China, is now also approved for treating adult patients with moderate to severe active rheumatoid arthritis (RA) who have had poor responses or intolerance to one or more TNF inhibitors.
Therapeutic Expansion
The expanded approval for ivarmacitinib highlights its potential in addressing unmet medical needs in rheumatoid arthritis patients. This JAK1 inhibitor offers a new therapeutic option for those who have not adequately responded to traditional TNF inhibitor therapies.
Market Opportunity
Rheumatoid arthritis affects a significant population globally, and the approval of ivarmacitinib provides Hengrui with a competitive edge in the immunology market. The company is well-positioned to capitalize on this opportunity with its robust R&D pipeline and market presence.-Fineline Info & Tech