China-based Sichuan Biokin Pharmaceutical Co., Ltd (SHA: 688506) has announced that it has secured four Phase II clinical trial approvals from the National Medical Products Administration (NMPA) for its in-house developed bispecific antibody drug conjugate (ADC) BL-B01D1. The approvals allow the drug to be tested in combination with other therapies, expanding its potential applications across various tumor types.
BL-B01D1: First-in-Class Innovation
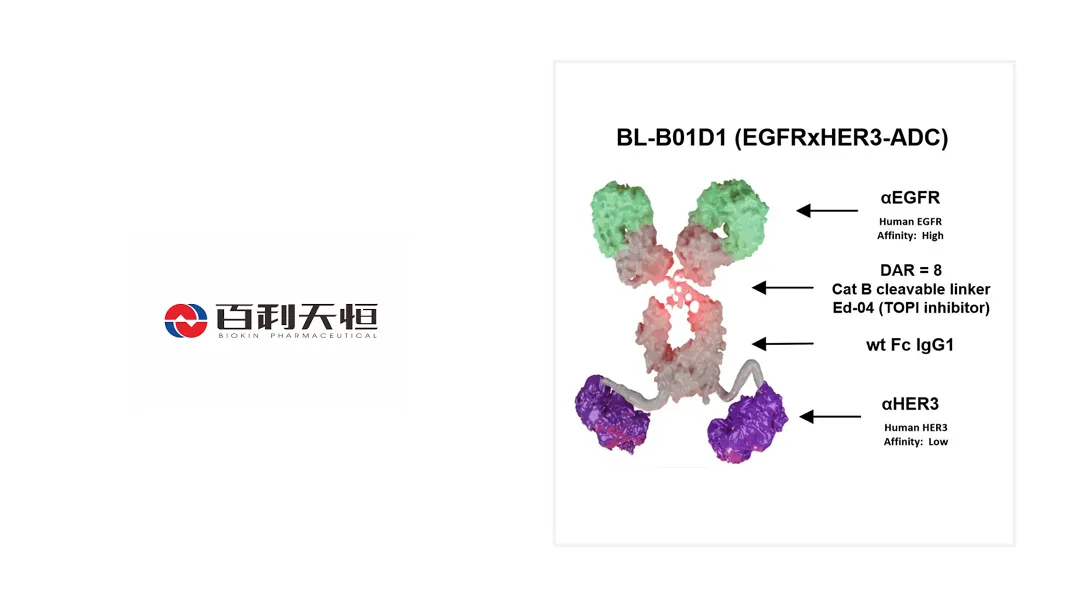
BL-B01D1 is the only first-in-class ADC targeting EGFR/HER3 that has reached Phase III clinical trials. It is currently subject to over 30 clinical studies across more than 10 tumor types in both China and the United States. This extensive clinical development underscores Biokin’s commitment to advancing innovative therapies for cancer patients.
Clinical Development and Future Outlook
The four new Phase II approvals further solidify Biokin’s position in the ADC market, enabling the company to explore BL-B01D1’s efficacy and safety in combination with other drugs. This strategic approach aims to enhance treatment outcomes for patients with diverse tumor types, including those with limited therapeutic options.-Fineline Info & Tech