Beijing-based biopharmaceutical company InnoCare Pharma (HKG: 9969, SHA: 688428) has announced that China’s Center for Drug Evaluation (CDE) has accepted the New Drug Application (NDA) for its next-generation pan-TRK inhibitor zurletrectinib (ICP-723). The proposed indication targets adult and adolescent patients (aged 12–18) with advanced solid tumors harboring NTRK gene fusions.
Zurletrectinib: Mechanism and Efficacy
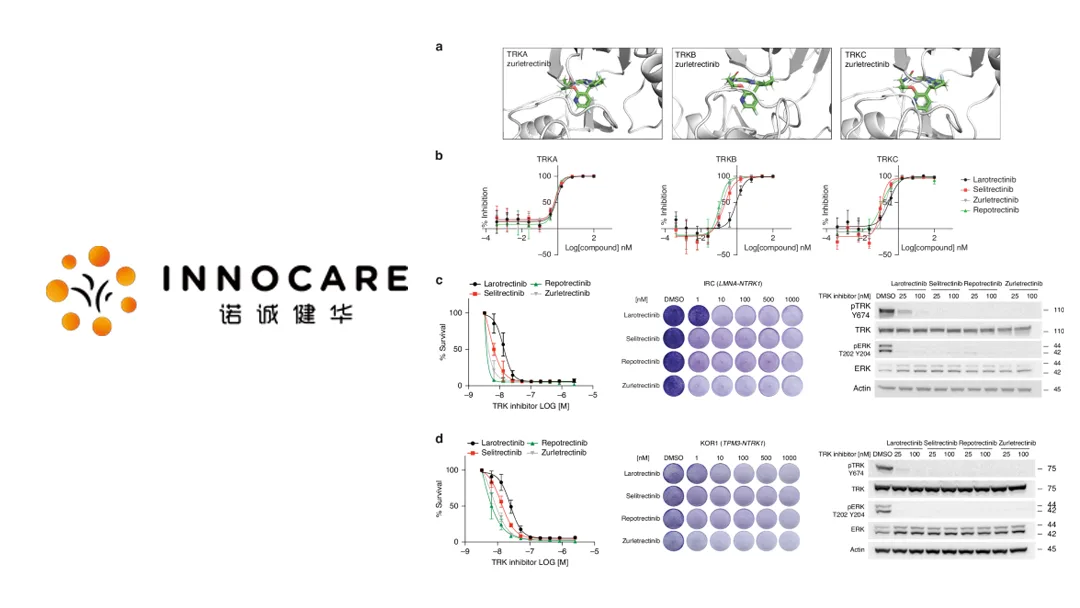
Zurletrectinib is a Category 1 drug designed to treat advanced or metastatic solid tumors with NTRK gene fusions, including those resistant to first-generation TRK inhibitors. Preclinical studies demonstrated outstanding efficacy and a favorable safety profile, positioning it as a promising therapeutic option for patients with limited treatment choices.
Clinical and Market Implications
NTRK gene fusions occur in various adult and pediatric tumors, with high incidence rates observed in rare cancers such as salivary gland carcinoma, secretory breast cancer, and infantile fibrosarcoma (exceeding 90% in some cases). The acceptance of the NDA by the CDE marks a significant step toward making zurletrectinib available to patients in China, potentially addressing critical unmet medical needs.-Fineline Info & Tech