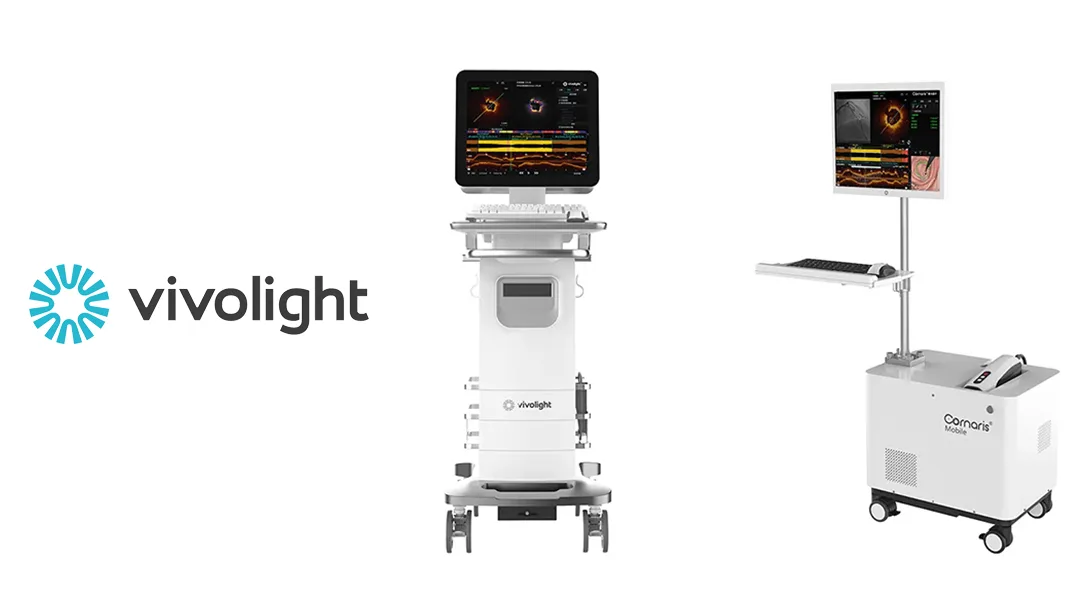
Shenzhen-based minimally invasive laser medical device firm Vivolight Medical Device & Technology Co., Ltd has obtained market clearance from the US Food and Drug Administration (FDA) for its multimodal coronary optical coherence tomography (OCT) system and accompanying catheter. This marks the first such approval globally for this advanced medical technology.
Company Background
Founded in 2012, Vivolight has established itself as a pioneer in the OCT industry. It became the first Chinese OCT manufacturer to enter overseas markets and currently holds the second-largest market share in China, following US-based Abbott Laboratories (NYSE: ABT).-Fineline Info & Tech