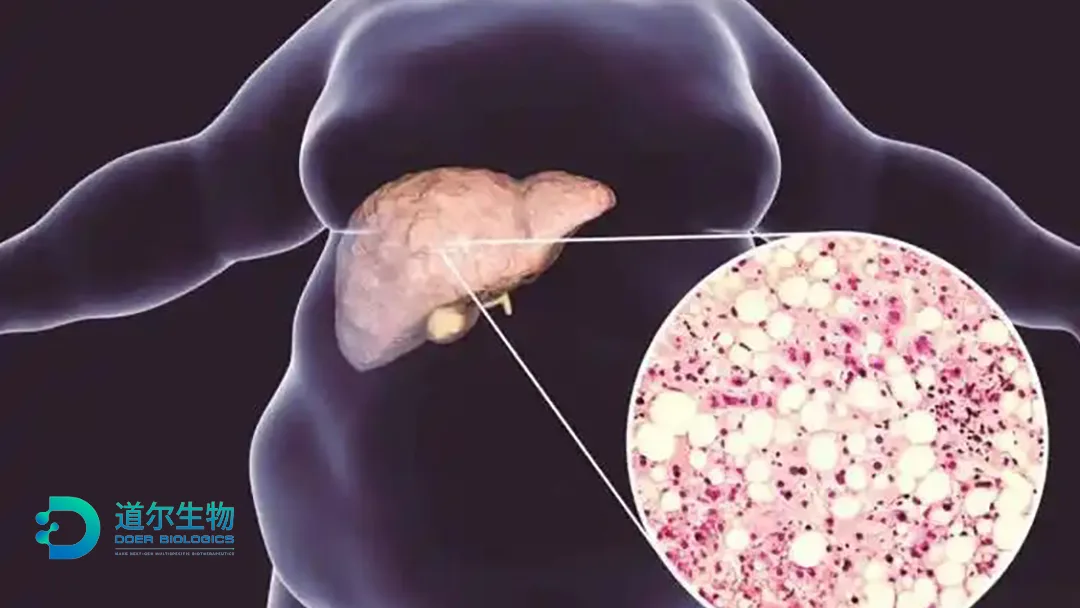
China-based Zhejiang Doer Biologics Co., Ltd has announced the first patient dosing in a Phase II study of DR10624, a novel tri-specific agonist targeting fibroblast growth factor 21 receptor (FGF21R), glucagon receptor (GCGR), and glucagon-like peptide-1 receptor (GLP-1R). The study focuses on metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH).
DR10624: First-in-Class Tri-Specific Agonist
DR10624 represents a new class of therapeutic agents designed to address MASLD and MASH. By simultaneously targeting FGF21R, GCGR, and GLP-1R, it aims to provide a comprehensive treatment approach for these conditions, which are closely linked to metabolic dysfunction and liver fibrosis.
Clinical Study Design
The Phase II study is a randomized, double-blinded, placebo-controlled trial evaluating the efficacy and safety of three dose levels of DR10624. It focuses on adult subjects at high risk of liver fibrosis associated with MASLD. The primary endpoint is the relative percentage change from baseline in liver fat content (LFC), as measured by MRI-PDFF after 12 weeks of treatment.-Fineline Info & Tech