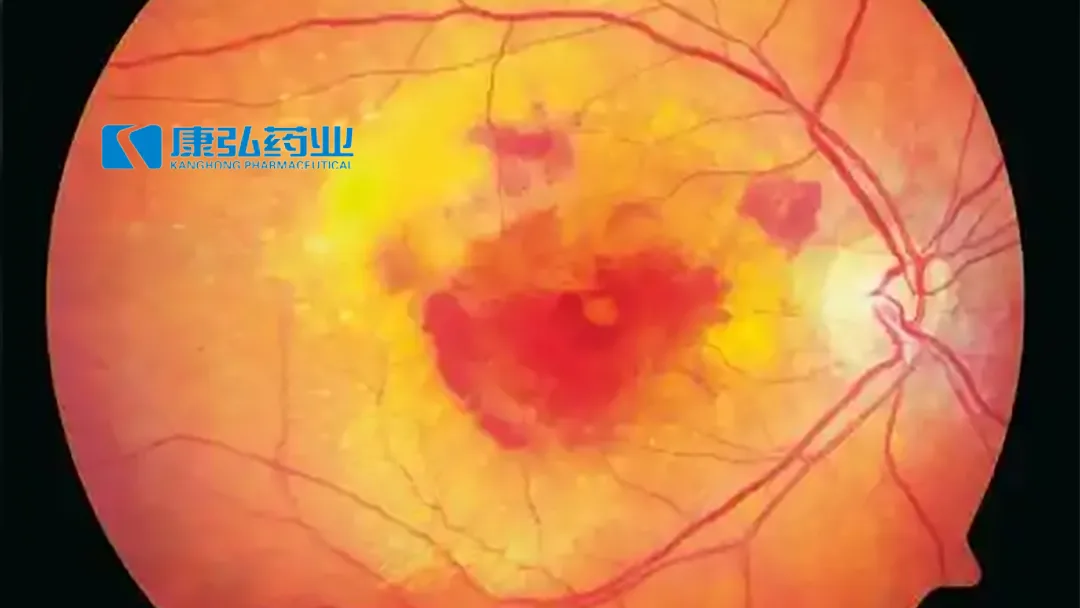
China-based Chengdu Origen Biotechnology Co., Ltd. announced the first patient dosing of KH658, a gene therapy co-developed by compatriot firm Chengdu Kanghong Pharmaceutical Group Co., Ltd.’s (SHE: 002773) US unit Vanotech Inc., in the dosage escalation Phase I VAN-2401 study for wet Age-related Macular Degeneration (wet AMD).
KH658 Mechanism and Preclinical Results
KH658 is a recombinant adeno-associated virus vector that encodes a human VEGF receptor fusion protein. In preclinical studies of wet AMD disease models, suprachoroidal space administration of KH658 resulted in the retention of the transgene product in the retina for prolonged periods and prevented disease progression. This innovative approach holds promise for addressing unmet medical needs in wet AMD treatment.-Fineline Info & Tech