China-based Lifetech Scientific Corporation (HKG: 1302) has announced that it has received marketing approval from the National Medical Products Administration (NMPA) for its aortic arch stent system. This innovative product was co-developed with Professor Shu Chang from the National Center for Cardiovascular Diseases and the Fuwai Hospital of the Chinese Academy of Medical Sciences.
Product Details and Innovation
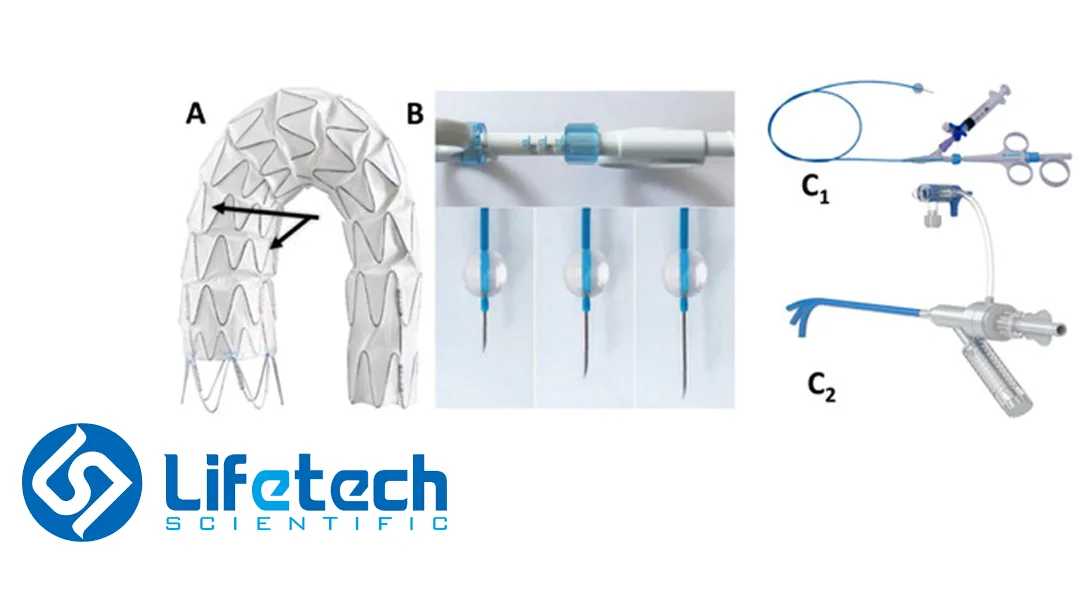
The aortic arch stent system is the first NMPA-approved covered stent specifically designed for partial reconstruction of the aortic arch using window opening technology. It comprises the Ankura Plus aortic arch main stent system and the CSkirt aortic arch branch stent system. The device is intended for endovascular repair treatment of Stanford B-type aortic dissection patients. It ensures precise maintenance of blood flow to the left subclavian artery while repairing diseased aortic vessels.
Clinical Outcomes and Benefits
Clinical studies have demonstrated excellent outcomes, with a 97.5% immediate technical success rate during surgery and a 99.1% branch vessel patency rate one year post-surgery. The incidence of Type III endoleaks was only 1.8% after one year, and no adverse events related to stent displacement occurred. This product provides a comprehensive solution for patients with insufficient anchoring areas or involvement of the left subclavian artery.-Fineline Info & Tech