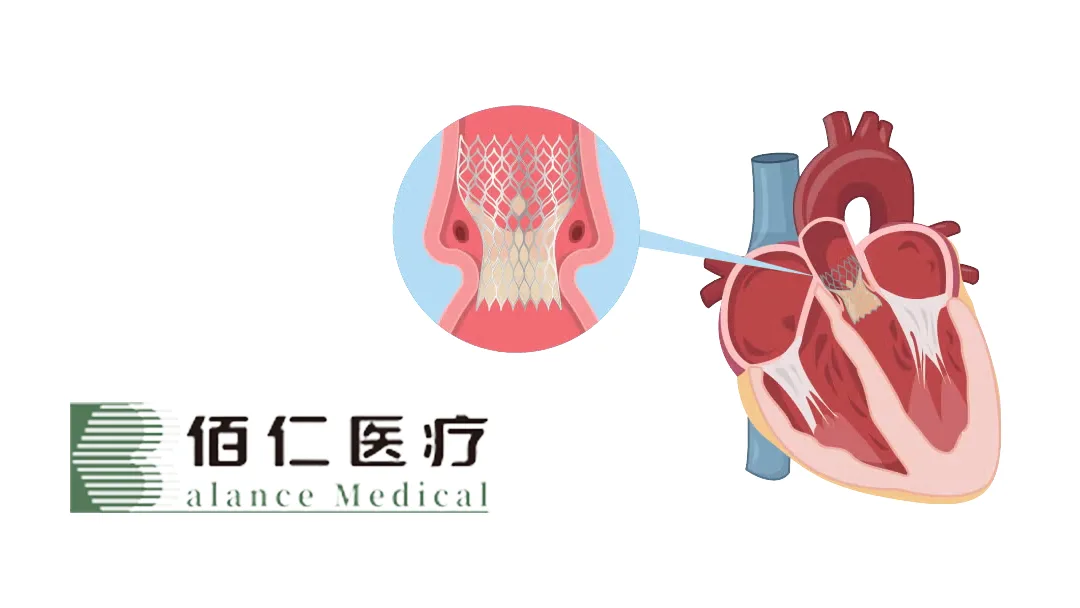
China’s Beijing Balance Medical Technology Co., Ltd (SHA: 688198) has obtained market authorization from China’s National Medical Products Administration (NMPA) for its Renato Transcatheter Valve-in-Valve System. This is the world’s first globally commercialized interventional device based on valve-in-valve (ViV) therapy, targeting high-surgical-risk patients with failed bioprosthetic valve implants. The system addresses conditions such as structural valve deterioration (SVD), prosthesis-patient mismatch (PPM), and valve thrombosis.
Product Design and Application
The Renato system is anatomically designed for artificial bioprosthetic valves. Its structure and characteristics enable secure anchoring at the inflow annulus of the bioprosthetic valve. Based on standardized circular inflow designs and inner diameter specifications of all implantable bioprosthetic valves, the product is designed in 2mm diameter increments to achieve precise valve-in-valve anchoring.
Patient benefits and lifecycle management
The system provides re-interventional treatment for patients with previously implanted bioprosthetic valves, effectively extending valve durability. It addresses concerns for younger Chinese patients opting for bioprostheses and accommodates diverse inner diameter requirements. This innovation complements the company’s previously launched limited-expansion bioprosthetic valve, enabling multiple re-interventional therapies and aligning with the clinical need for comprehensive lifecycle management of valvular heart disease.-Fineline Info & Tech