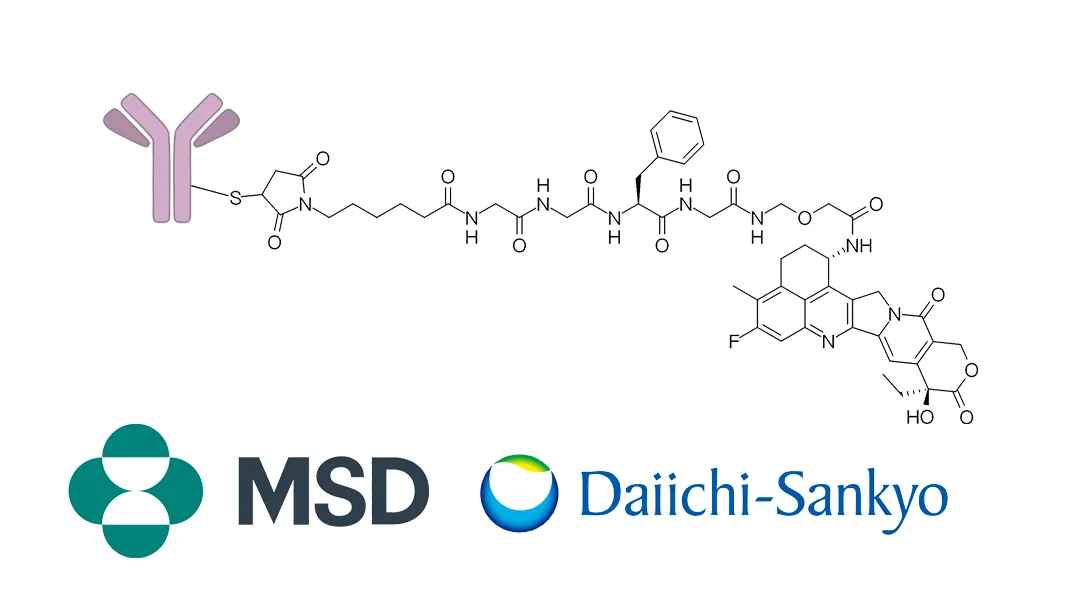
US-based Merck, Sharp & Dohme Inc. (MSD; NYSE: MRK) announced the first patient dosing in the Phase III IDeate-Esophageal01 study of ifinatamab deruxtecan (I-DXd). This potential first-in-class B7-H3 directed DXd antibody drug conjugate (ADC) is co-developed with Japan-based Daiichi Sankyo (TYO: 4568). The trial will assess the efficacy and safety of I-DXd versus investigator’s choice of chemotherapy in patients with unresectable advanced or metastatic esophageal squamous cell carcinoma (ESCC).
Clinical Trial Details
The IDeate-Esophageal01 study targets patients with disease progression following treatment with a platinum-containing systemic therapy and an immune checkpoint inhibitor. This Phase III trial builds on the positive results from the Phase I/II IDeate-PanTumor01 study, which demonstrated significant efficacy of I-DXd in heavily pretreated patients with ESCC.-Fineline Info & Tech