BeiGene, Ltd. (NASDAQ: ONC, HKG: 6160, SHA: 688235), a China-based oncology company proposing to rename itself BeOne Medicines Ltd., announced positive results from the Phase II clinical trial DeLLphi-307 (NCT06502977) for tarlatamab, conducted in collaboration with US biotech Amgen (NASDAQ: AMGN) in China. The trial highlights the drug’s potential in treating extensive-stage small cell lung cancer (ES-SCLC).
Tarlatamab Mechanism and Development
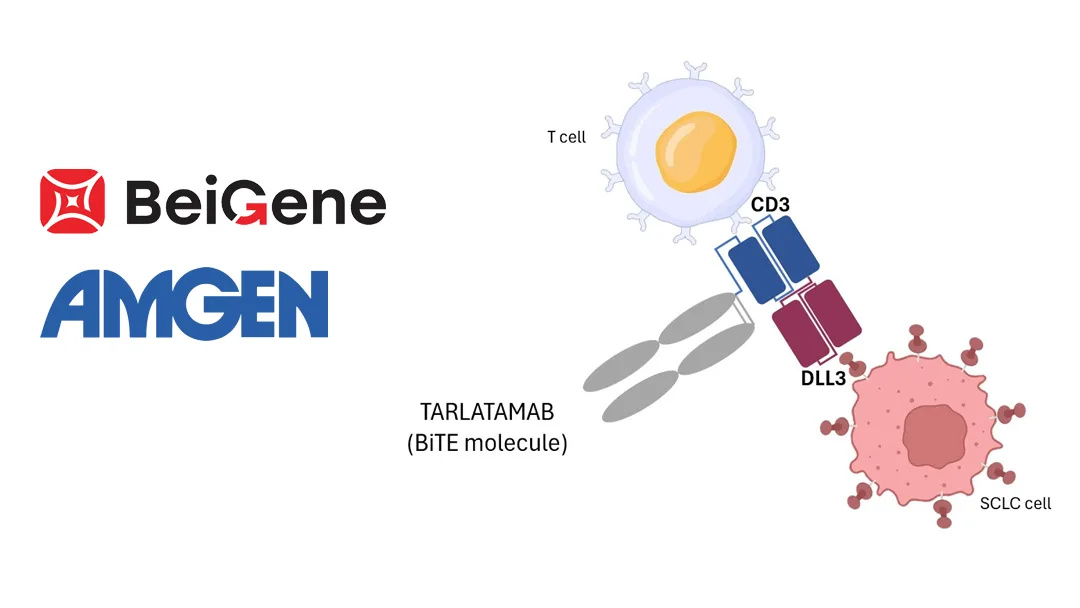
Tarlatamab is a bispecific T-cell engager (BiTE) targeting DLL3 and CD3, designed to bind to DLL3 on tumor cells and CD3 on T cells. Through its global oncology collaboration with Amgen, BeiGene is co-developing and commercializing tarlatamab in China, with plans to submit a marketing application for relevant indications within the year.
Clinical Trial Details
The DeLLphi-307 trial assessed the efficacy, safety, and tolerability of tarlatamab in Chinese patients with ES-SCLC who had failed at least two prior lines of therapy, including platinum-based chemotherapy. The primary endpoint of the trial was objective response rate (ORR). Detailed data from the trial will be presented at an upcoming medical conference.
Previous Trial Results
Earlier Phase II (DeLLphi-301) and Phase III (DeLLphi-304) trials of tarlatamab for second-line SCLC treatment also demonstrated positive results. Based on data from DeLLphi-301, the U.S. FDA granted tarlatamab accelerated approval for this indication.-Fineline Info & Tech