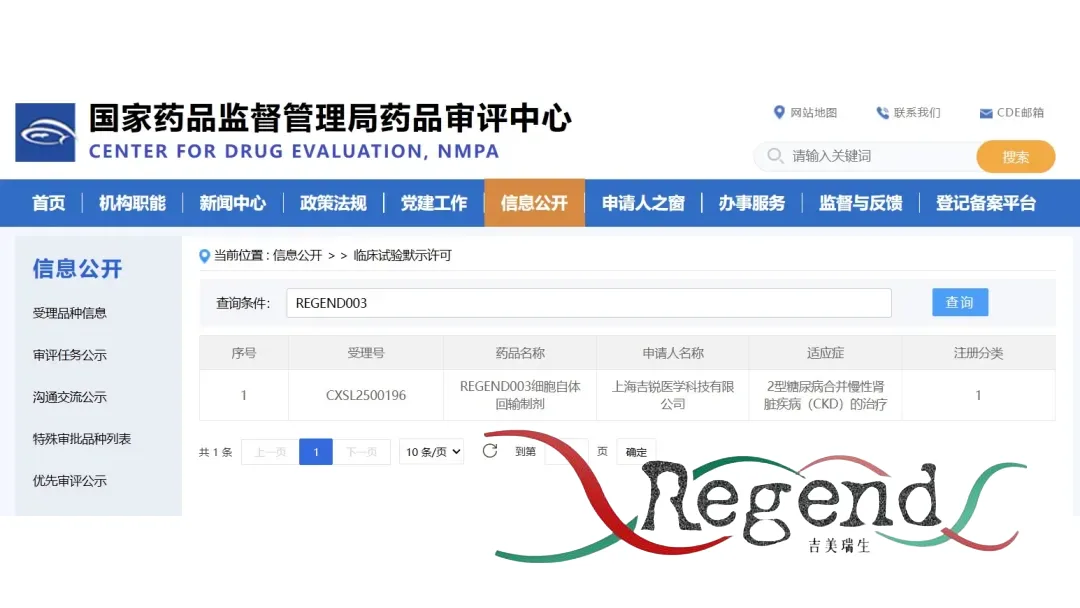
Suzhou-based stem cell therapy specialist Regend Therapeutics announced that it has received approval from China’s National Medical Products Administration (NMPA) to proceed with a Phase I/II clinical study of its REGEND003 therapy. This autologous renal progenerator stem cell therapy targets patients with type 2 diabetes and chronic kidney disease (CKD), addressing a significant unmet medical need.
REGEND003: A First-in-Class Renal Stem Cell Therapy
REGEND003 represents a groundbreaking approach as the first renal stem cell therapy to enter clinical development globally. The therapy utilizes SOX9⁺CD73⁺ renal precursor cells derived from a patient’s own kidneys to promote renal tissue regeneration and functional reconstruction of nephrons. By isolating target cells from patient urine samples in a non-invasive manner, REGEND003 repopulates damaged renal structures and secretes regenerative factors to restore tubular and glomerular function.
Advantages of the Novel Approach
Compared with traditional biopsy methods, REGEND003 offers a lower-risk, repeatable sampling approach and scalable manufacturing. This innovative method highlights Regend Therapeutics’ commitment to advancing stem cell-based treatments for complex conditions such as diabetes-related chronic kidney disease.-Fineline Info & Tech