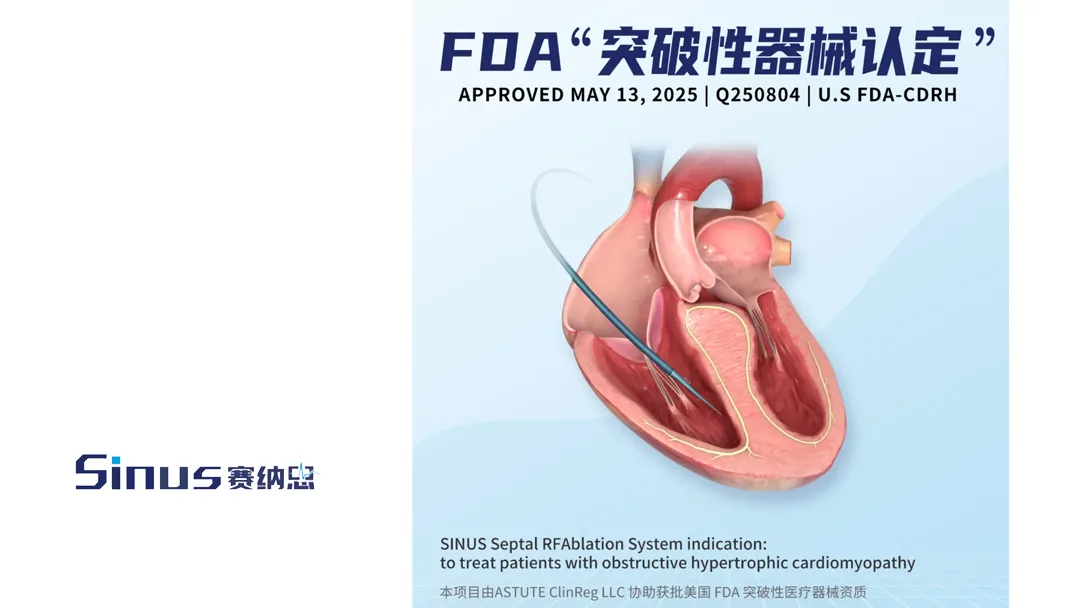
Suzhou Sinus Medical Technologies Co., Ltd., a China-based medical device innovator, announced that its in-house developed interventricular radiofrequency ablation system has received the Breakthrough Device Designation (BDD) from the US Food and Drug Administration (FDA). This recognition is for the treatment of obstructive hypertrophic cardiomyopathy (oHCM), a condition characterized by thickened heart muscle that can impair blood flow and lead to serious cardiovascular complications.
Company Overview
Sinus Medtech is dedicated to developing cutting-edge devices for the management of major cardiovascular diseases. Its robust product pipeline is particularly focused on solutions for heart failure and atrial fibrillation, addressing significant unmet clinical needs in the cardiovascular space.
Device and Innovation
The interventricular radiofrequency ablation system is designed to provide a minimally invasive treatment option for patients with oHCM. By leveraging advanced radiofrequency technology, the system aims to precisely target and ablate overgrown heart muscle tissue, thereby improving blood flow and reducing symptoms associated with the condition.-Fineline Info & Tech