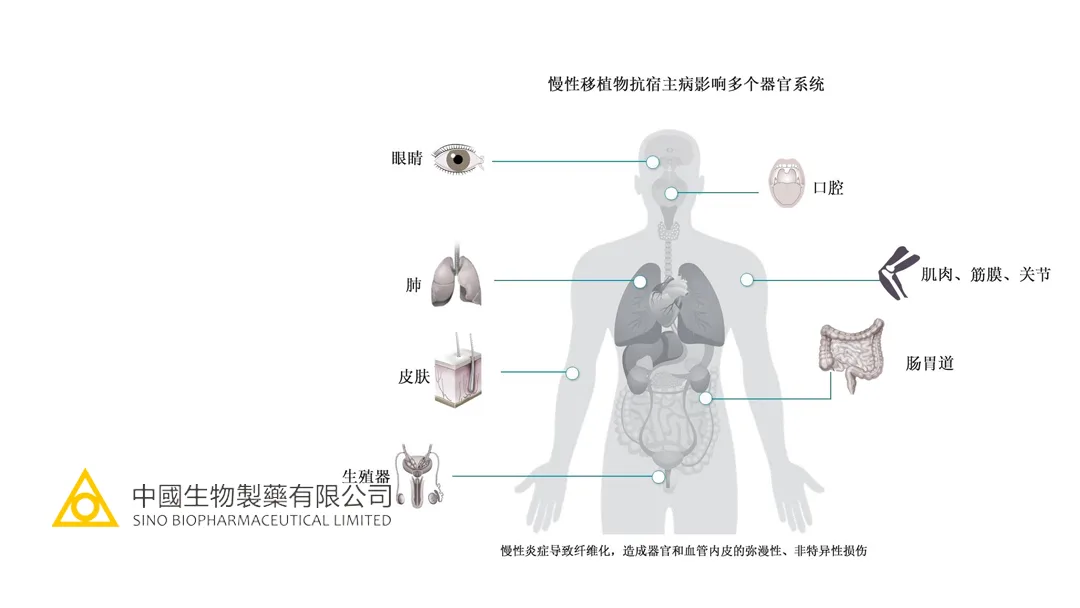
China-based Sino Biopharmaceutical Ltd (HKG: 1177) announced that its Class 1 drug TDI01 has been included in the Breakthrough Therapy Designation (BTD) program by the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA). The designation covers its use in treating moderate-to-severe chronic graft-versus-host disease (cGVHD) in patients who have undergone 1 to 5 prior lines of systemic therapy.
Mechanism of Action
TDI01 is a novel, oral, and highly selective ROCK2 inhibitor. It effectively suppresses pro-inflammatory Th17 cells and promotes regulatory T cells (Tregs), restoring immune homeostasis. Additionally, it targets fibroblast ROCK2, blocking their differentiation into myofibroblasts and inducing apoptosis of existing myofibroblasts. This dual mechanism allows for immune regulation and fibrosis reversal.
Clinical Progress
Previously, TDI01 was cleared in China for clinical trials targeting idiopathic pulmonary fibrosis (IPF), pneumoconiosis, and liver fibrosis.-Fineline Info & Tech