China-based Huadong Medicine Co., Ltd (SHE: 000963) announced that it has received clinical trial approval from the National Medical Products Administration (NMPA) for its category 1 biologic drug HDM2020. This marks a significant milestone in the development of the company’s innovative oncology therapy.
Drug Profile
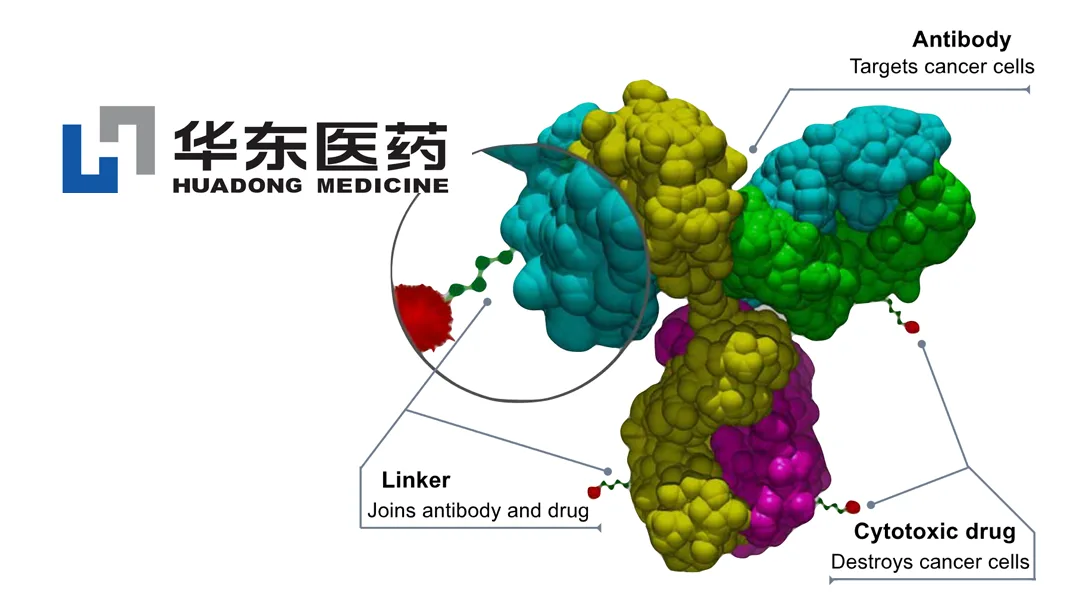
HDM2020 is an antibody-drug conjugate (ADC) targeting fibroblast growth factor receptor 2b (FGFR2b). It is designed for the treatment of advanced solid tumors and represents a novel approach to addressing unmet medical needs in oncology.
Preclinical Results
The drug has demonstrated potent antitumor activity in FGFR2b-positive preclinical models, including gastric cancer and squamous non-small cell lung cancer (sqNSCLC). These results highlight its strong druggability and favorable safety profile, positioning it as a promising candidate for further clinical evaluation.-Fineline Info & Tech