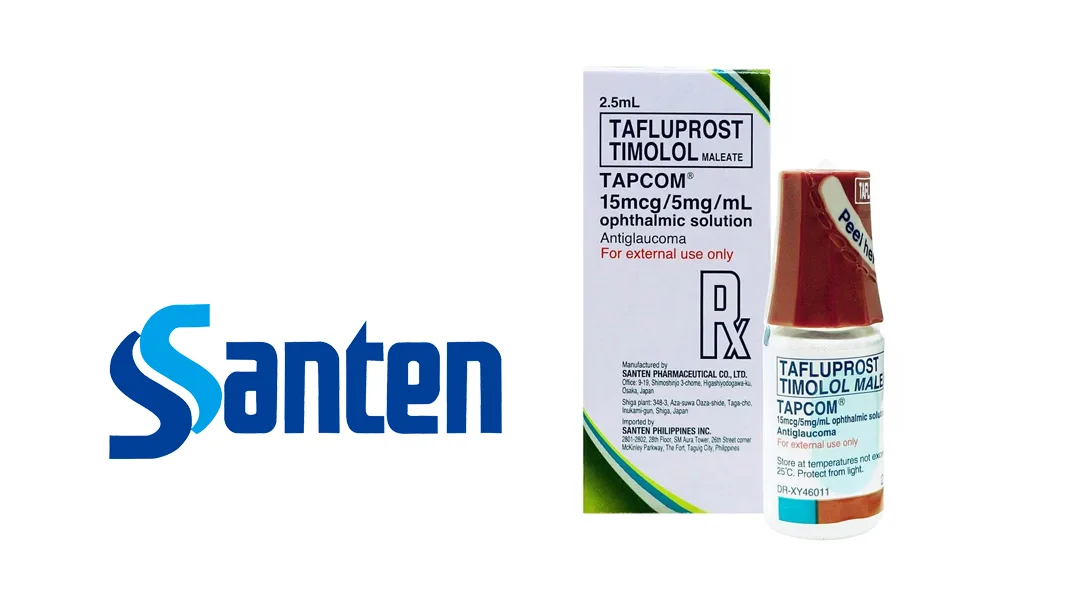
Japan-based Santen Pharmaceutical Co., Ltd. announced that its novel glaucoma treatment, Tapcom (tafluprost/timolol maleate), has been administered to the first patient at the Zhongshan Ophthalmic Center of Sun Yat-sen University in the Guangdong Hong Kong Macao Greater Bay Area (GBA). This milestone was achieved under the “Hong Kong-Macao Drug and Device Access” policy.
Drug Approval and Indication
Tapcom, indicated for reducing intraocular pressure (IOP) in adults with open-angle glaucoma or ocular hypertension, was approved in Guangdong via the “Hong Kong-Macao Drug and Device Access” policy in early 2025. The treatment is suitable for patients who show inadequate response to monotherapy with either beta-blockers or prostaglandin analogs.
Product Advantages
The drug features a preservative-free formulation to enhance patient benefits.-Fineline Info & Tech