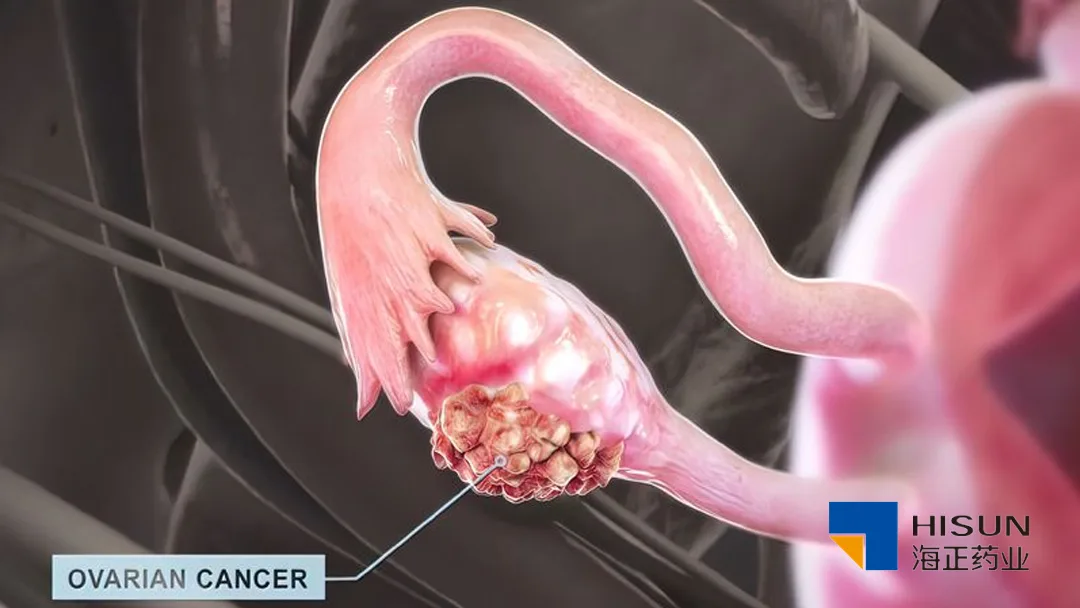
China-based Zhejiang Hisun Pharmaceutical Co., Ltd (SHA: 600267) announced that it has received clinical trial approval from the National Medical Products Administration (NMPA) for its Category 1 chemical drug, HS387. This milestone paves the way for the drug’s development in treating advanced solid tumors.
HS387: A Novel KIF18A Inhibitor
HS387, an in-house developed selective KIF18A inhibitor, is designed for the treatment of high-grade serous ovarian cancer (HGSOC), non-small cell lung cancer (NSCLC), and other advanced solid tumors. While several KIF18A inhibitors have entered clinical trials globally, there are currently no approved products in this class, highlighting the potential of HS387 to address unmet medical needs.-Fineline Info & Tech