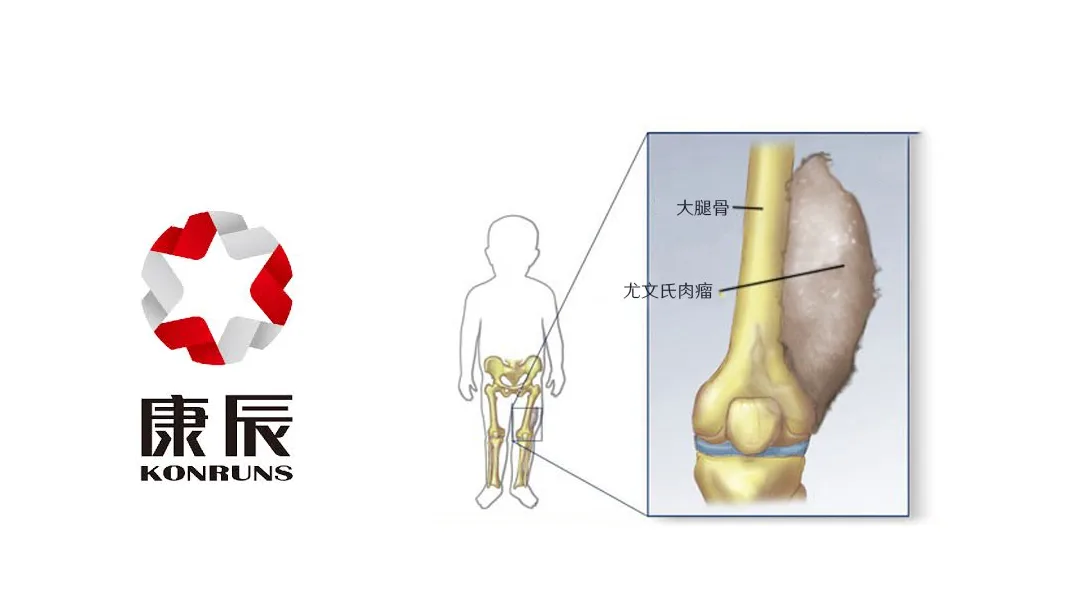
Beijing-based Konruns Pharmaceutical Co., Ltd. (SHA: 603590) announced on Wednesday that China’s Center for Drug Evaluation (CDE) has proposed adding its oral kinase inhibitor KC1036 to the Support Anti-tumor drugs R&D for Kids (SPARK) pilot program. This initiative aims to accelerate the development of treatments for pediatric Ewing’s sarcoma. The public comment period for this proposal closes on July 23.
About KC1036
KC1036 is a Category 1.1 small-molecule drug discovered in-house by Konruns. It works by simultaneously blocking VEGFR2 and AXL, thereby cutting off the blood supply to tumors and inhibiting their ability to evade the immune system. Konruns holds global intellectual property rights for this promising asset.
SPARK Pilot Program
The SPARK pilot program is an extension of the patient-centric CARE scheme for rare diseases. It implements the National Medical Products Administration’s (NMPA) mandate of “early engagement, one-company-one-policy, end-to-end guidance, integrated review.” The program offers sponsors tailored support on development strategy, trial design, pediatric formulations, and dosing. This support is designed to shorten development timelines and improve access to medicines for rare childhood cancers.-Fineline Info & Tech