VivaVision Biotech recently announced the successful closing of its D2+ funding round, raising 140 million RMB. The round was led by Wenzhou Ouhai Xiangtou Yicun Capital (Limited Partnership), with participation from new investors such as Guangdong Technology Financial Group and Renze Zhenhe. Existing shareholders Lapam Capital and Yicun Capital also increased their support.
Strategic Advancement and Local Support
Since implementing its strategy in Wenzhou Ouhai Gene Valley at the end of 2023, VivaVision Biotech has received strong policy and industrial resource support from the local area. The company’s core pipeline has entered mid-to-late clinical stages, marked by several key successes:
- VVN461LD: Received positive preliminary written feedback from the FDA, requiring only one Phase III pivotal clinical study to treat postoperative ophthalmic inflammation before filing for NDA.
- VVN461HD: Included in China’s National Medical Products Administration “List of Breakthrough Therapy Drugs,” positioning it for potential accelerated review and approval.
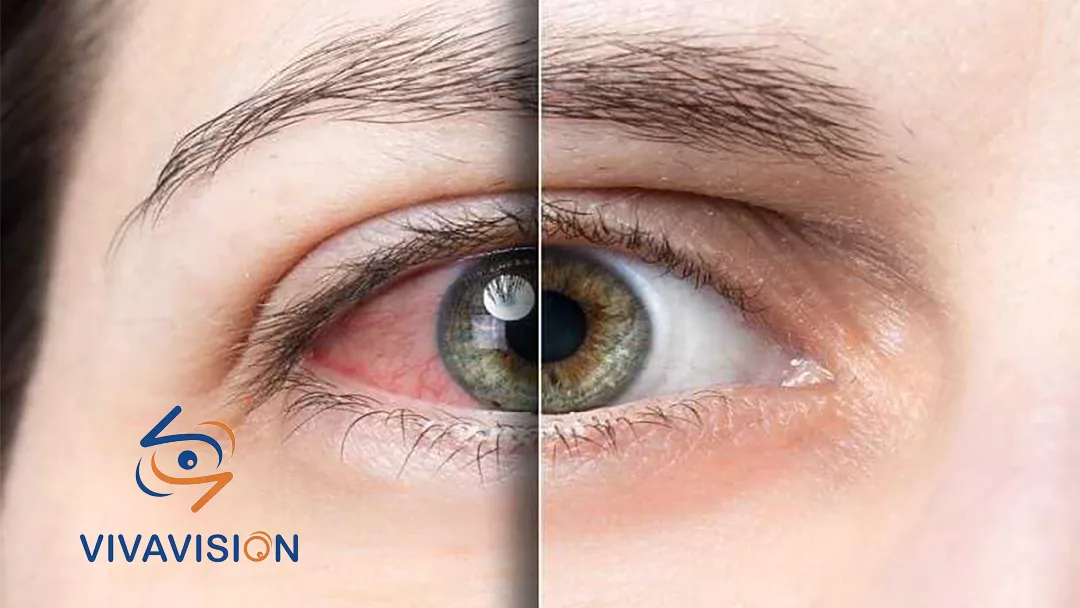
- VVN001: A new generation of domestic dry eye syndrome eye drops, steadily advancing in Phase III clinical trials.
- VVN1901: For the treatment of neurotrophic keratitis, recently enrolled its first patient in Phase II clinical trials, officially entering the key clinical stage.-Fineline Info & Tech