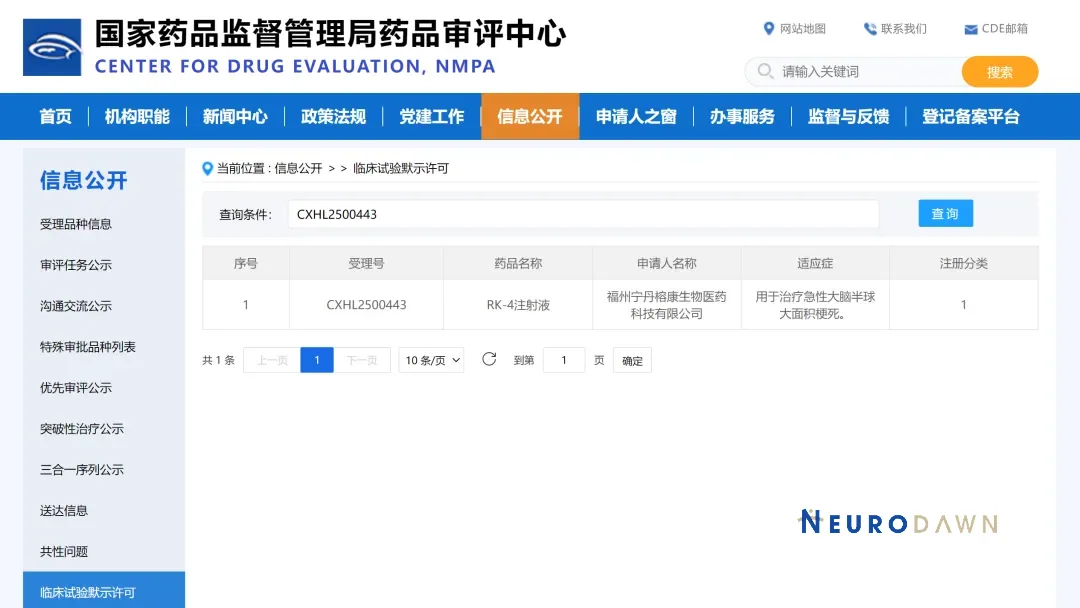
China-based Neurodawn Pharmaceutical Co., Ltd.’s wholly-owned subsidiary, Fuzhou Ningdan Rongkang Biomedical Technology Co., Ltd. (Ningdan Rongkang), announced that its self-developed Class 1 chemical new drug, RK-4 Injection, has received clinical trial approval from China’s National Medical Products Administration (NMPA) under approval number 2025LP01893. This marks a significant advancement in the treatment of acute large hemispheric infarction (LHI), a condition with substantial unmet clinical needs.
RK-4 Injection: A Novel Treatment Approach
RK-4 Injection represents the world’s first innovative drug for treating LHI via transcranial bone marrow cavity injection. This unique administration route allows the drug to rapidly enter the brain parenchyma and effectively distribute to the lesion site. By bypassing traditional delivery challenges, RK-4 Injection offers a promising new therapeutic option for patients suffering from this severe form of stroke.
Addressing Unmet Clinical Needs
LHI is characterized by significant disability and mortality due to the lack of effective treatment options. The approval of RK-4 Injection highlights the potential to address this critical gap in clinical care. Ningdan Rongkang’s commitment to developing innovative solutions for neurological disorders has led to this milestone achievement.
Clinical Development Plans
Ningdan Rongkang plans to initiate clinical studies for RK-4 Injection shortly. This phase will focus on evaluating the safety and efficacy of the drug in LHI patients, with the goal of advancing toward broader clinical application and potential market approval.-Fineline Info & Tech