China-based BeOne Medicines Ltd. (NASDAQ: ONC, HKG: 6160, SHA: 688235) announced on July 31, 2025, that its investigational Bruton’s tyrosine kinase (BTK) degrader, BGB-16673, has been granted PRIority MEdicines (PRIME) designation by the European Medicines Agency (EMA) for the treatment of patients with Waldenström’s macroglobulinemia (WM) who have previously received a BTK inhibitor. This marks the company’s first PRIME designation, highlighting the potential of BGB-16673 to address a critical unmet medical need.
EMA’s Positive Opinion on Orphan Drug Designation
The EMA’s Committee for Medicinal Products for Human Use (CHMP) has also issued a positive opinion on the orphan drug designation application for BGB-16673 for WM patients. A final decision is expected within weeks, which could further expedite the development and approval process for this innovative therapy.
FDA Fast Track Designation
In addition to the EMA’s PRIME designation, BGB-16673 has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA) for adult patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL). This dual recognition underscores the drug’s potential to significantly impact the treatment landscape for these hematologic malignancies.
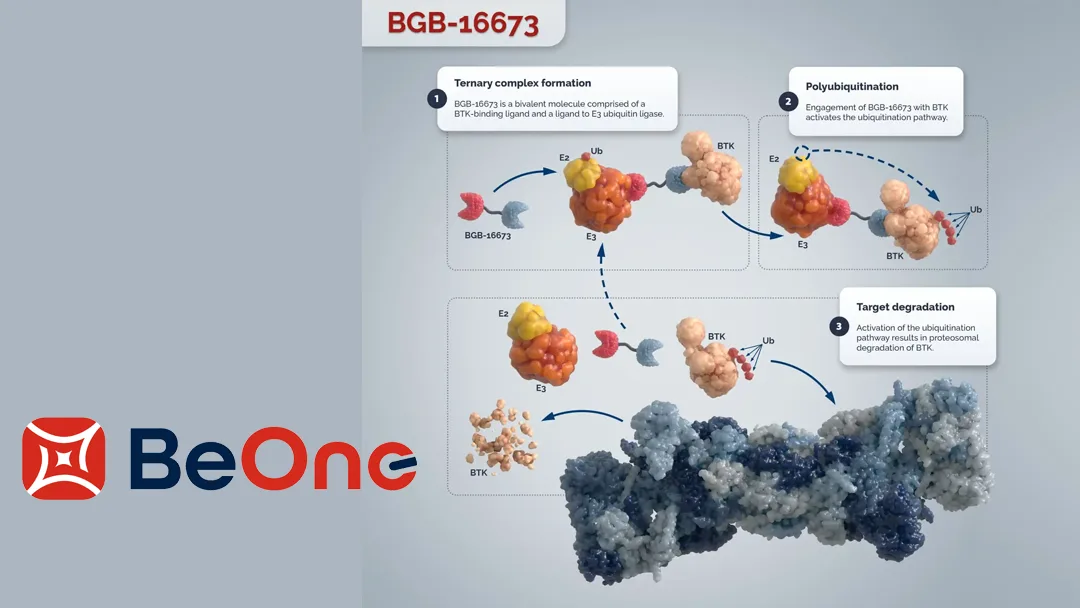
Innovative Mechanism of BGB-16673
BGB-16673 is an oral BTK protein degrader developed using BeOne’s proprietary Chimeric Degradation Activating Compound (CDAC) platform. It is designed to overcome resistance to BTK inhibitors by targeting both wild-type and multiple drug-resistant mutant BTK proteins. The CHMP noted the drug’s innovative mechanism and compelling preclinical data, particularly its ability to address the limited treatment options for WM patients who have previously received BTK inhibitors.-Fineline Info & Tech