Sichuan Biokin Pharmaceutical Co., Ltd (SHA: 688506) announced that its bispecific antibody-drug conjugate (ADC), izalontamab brengitecan (iza-bren), co-developed with Bristol-Myers Squibb (BMS; NYSE: BMY), has been granted Breakthrough Therapy Designation (BTD) by the U.S. Food and Drug Administration (FDA). This designation is for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring EGFR exon 19 deletions or exon 21 L858R substitution mutations. These patients have previously failed EGFR-TKI therapy and platinum-containing chemotherapy. Additionally, the Phase 3 clinical trial of iza-bren in patients with platinum-resistant recurrent epithelial ovarian cancer has recently completed enrollment of its first participant.
Product Profile
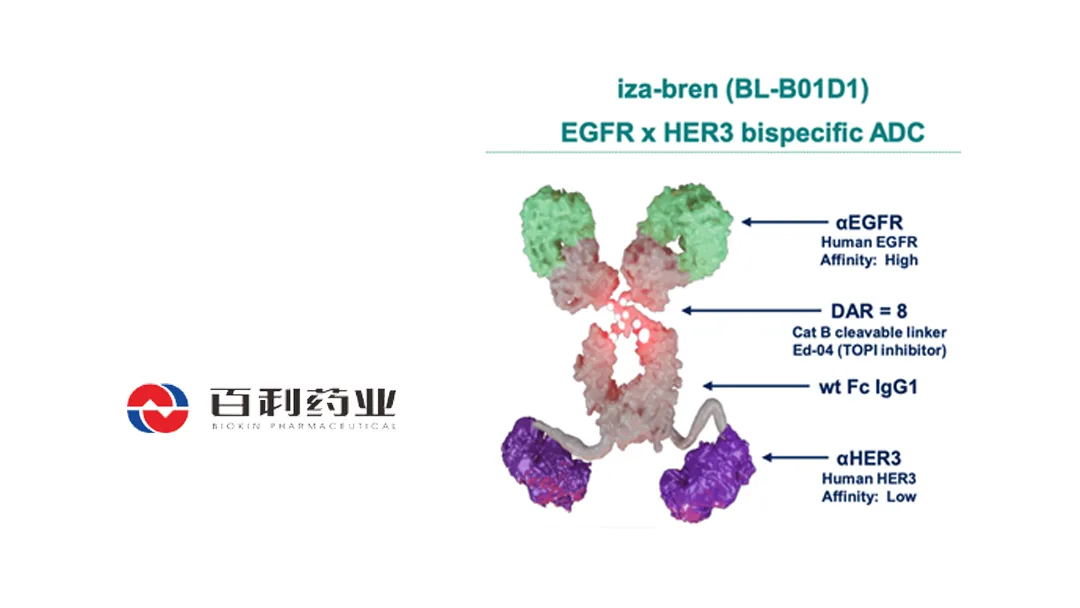
Iza-bren is a first-in-class, EGFR×HER3 bispecific ADC. It is the first and currently the only bispecific ADC in Phase 3 clinical development. The drug is undergoing over 40 clinical trials targeting various tumor types in both China and the United States.
Regulatory Milestones
To date, iza-bren has been included in the Breakthrough Therapy Designation list by the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) for five indications.-Fineline Info & Tech