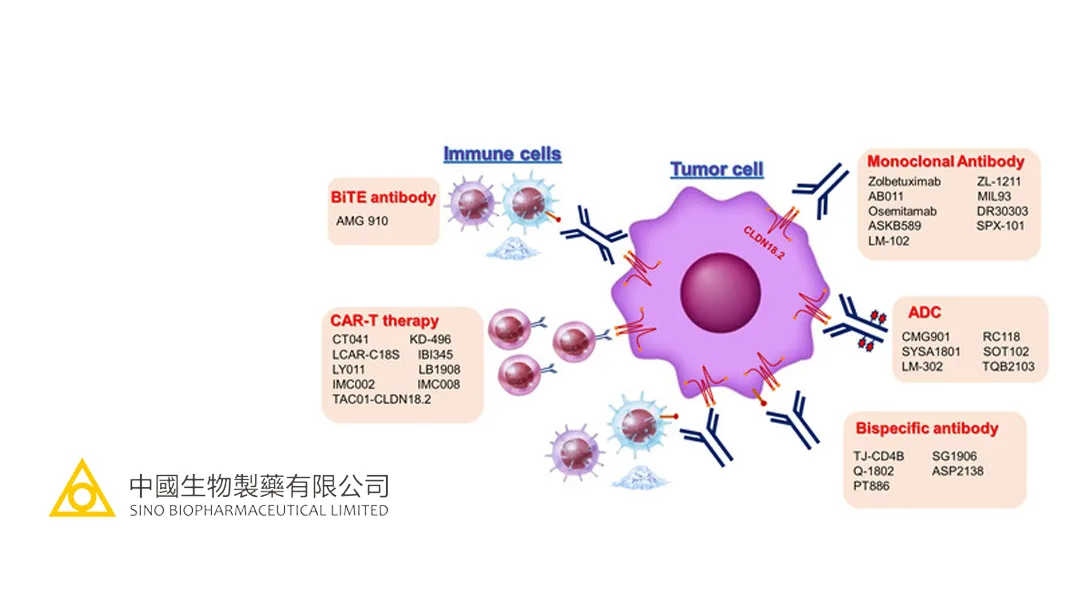
China-based Sino Biopharmaceutical Ltd (HKG: 1177) announced that LM-302, an innovative antibody-drug conjugate (ADC) developed by its subsidiary LaNova Medicines Ltd, has been granted Breakthrough Therapy Designation by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA). This designation is for LM-302’s use in combination with a PD-1 monoclonal antibody for the first-line treatment of Claudin 18.2-positive locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma.
Clinical Efficacy and Data
LM-302, a potential first-in-class ADC targeting Claudin 18.2, has demonstrated clinical efficacy in patients with gastric, pancreatic, and bile duct cancers, including those with low expression of Claudin 18.2 and PD-L1. At the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, LaNova presented new data on LM-302 in combination with a PD-1 monoclonal antibody for gastric cancer. Among 41 evaluable patients, the overall response rate (ORR) was 65.9%, and the disease control rate (DCR) was 85.4%. For the 32 patients with Claudin 18.2 expression of at least 25%, the ORR was 71.9% and the DCR was 96.9%. These results highlight LM-302’s promising anti-tumor activity and manageable safety profile in Claudin 18.2-positive patients.
Phase III Clinical Trial
LM-302 is currently undergoing a Phase III clinical trial in China for the treatment of Claudin 18.2-positive locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma that has progressed after two or more lines of systemic therapy. This trial aims to further evaluate the efficacy and safety of LM-302 in this patient population, building on the positive data presented at ASCO.-Fineline Info & Tech