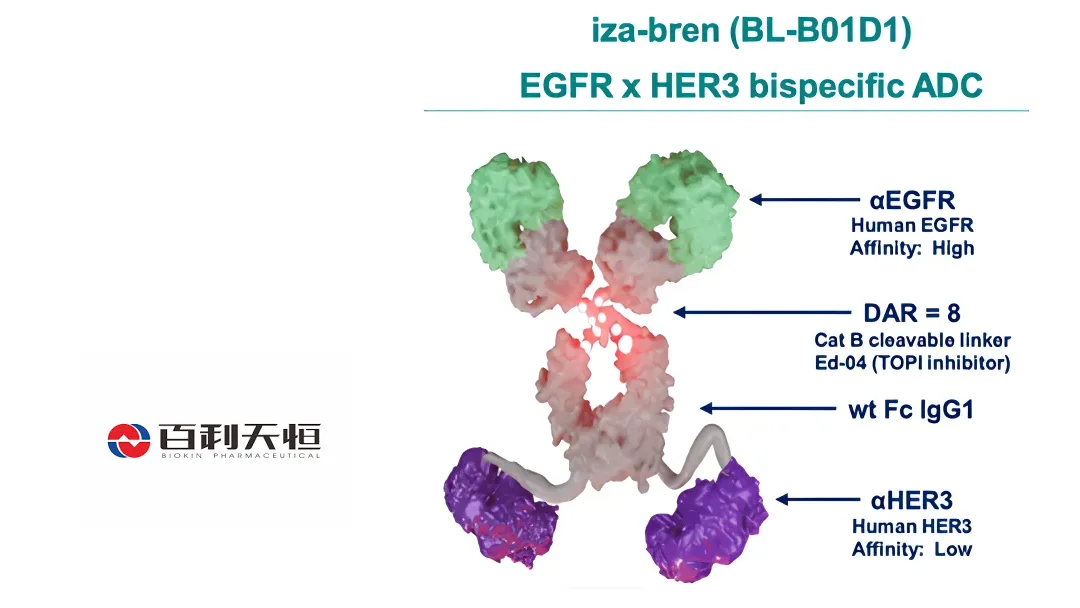
China‑based Sichuan Biokin Pharmaceutical Co., Ltd. (SHA: 688506) announced today that its bispecific antibody‑drug conjugate (ADC), izalontamab brengitecan (iza‑bren, code: BL‑B01D1), has received Breakthrough Therapy Designation (BTD) from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA).
The designation targets platinum‑resistant recurrent epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer, positioning izalontamab as a first‑in‑class therapy in a high‑unmet‑need patient population.
Co‑Development and Global Rights
izalontamab is an EGFR × HER3 bispecific ADC and the only candidate of its kind in Phase 3 clinical development worldwide.
In December 2023, Biokin Pharma signed a licensing and collaboration agreement with Bristol‑Myers Squibb (BMS, NYSE: BMY). Under the deal, BMS receives global development and commercialization rights outside mainland China, while Biokin retains all rights within China.
Clinical Pipeline and Market Impact
- Clinical Trials: Over 40 active trials across China and the United States span multiple tumor types.
- Phase 3 Status: The BTD is expected to accelerate the pivotal study schedule, potentially shortening the time to regulatory submission.
- Strategic Advantage: The partnership combines Biokin’s domestic market expertise with BMS’s global commercialization capabilities, creating a unique pathway for a novel ADC in the oncology landscape.
Outlook
The BTD designation is a significant milestone for both companies, underscoring the therapeutic promise of bispecific ADCs in gynecologic oncology. If the Phase 3 program meets its endpoints, izalontamab could become a new standard of care for patients with platinum‑resistant disease, driving substantial growth for Biokin in China and for BMS in international markets.-Fineline Info & Tech