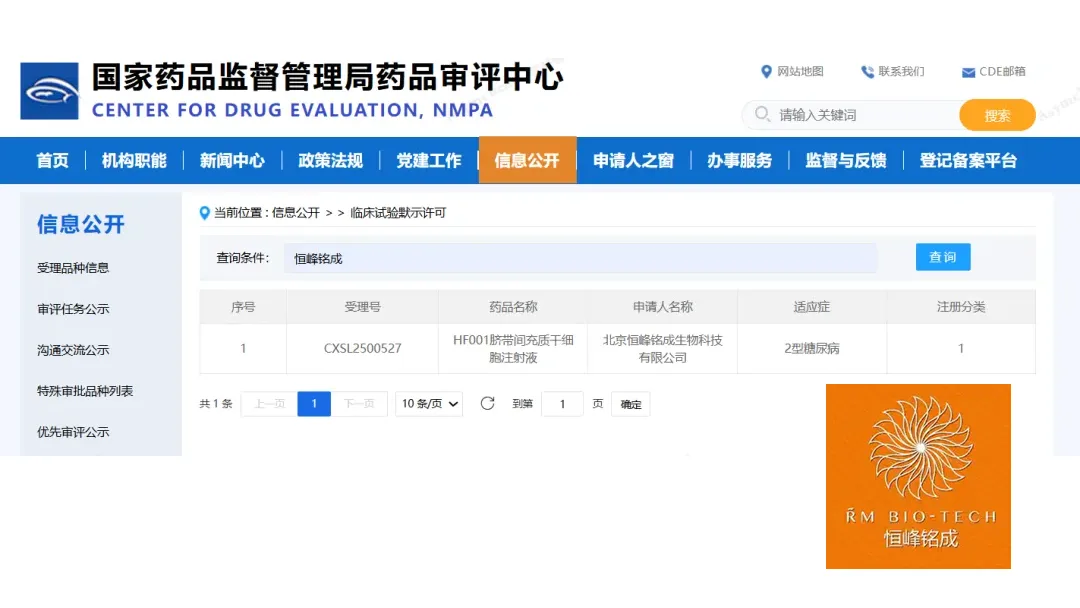
Beijing RM Bio‑Tech Co., Ltd. announced that the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA) has approved its Investigational New Drug (IND) application for HF001, an umbilical‑cord‑mesenchymal‑stem‑cell (UC‑MSC) injection, for the treatment of Type 2 diabetes (T2D).
Product Profile
- HF001 is a fully‑humanised, GMP‑grade UC‑MSC therapy derived from healthy donor umbilical‑cord tissue.
- The product is engineered to restore insulin‑secreting β‑cell mass and enhance peripheral insulin sensitivity, thereby reducing exogenous insulin requirements.
- Manufacturing leverages a scalable bioreactor platform, positioning RM Bio‑Tech for rapid global expansion.
Clinical Evidence
| Phase | Sample Size | Key Outcomes (48 Weeks) | Clinical Significance |
|---|---|---|---|
| Phase I/II | 120 | HbA1c reduction: 1.2 % ± 0.3 % | Demonstrates meaningful glycaemic improvement with a favourable safety profile. |
| Phase II | 60 | Daily insulin dose: ↓ 45 % | Indicates potential for substantial cost savings and improved patient quality of life. |
| Safety | — | No serious adverse events | Confirms the tolerability of UC‑MSC infusion. |
Regulatory Milestone
- CDE IND clearance removes the final regulatory hurdle before multinational phase‑III trials, slated to commence in Q1 2026.
- The approval aligns with China’s “Made in China 2025” initiative, prioritising innovative biotherapeutics and reinforcing RM Bio‑Tech’s position as a domestic leader in regenerative medicine.
Market Implications
- The global T2D therapeutics market is projected to surpass $200 billion by 2030; stem‑cell therapies represent a high‑growth sub‑segment.
- HF001’s unique mechanism—direct β‑cell regeneration versus pharmacologic agents—offers a differentiated value proposition to clinicians and payers.
- RM Bio‑Tech is actively pursuing strategic collaborations with diabetes‑care platforms to accelerate co‑development, licensing, and distribution worldwide.-Fineline Info & Tech