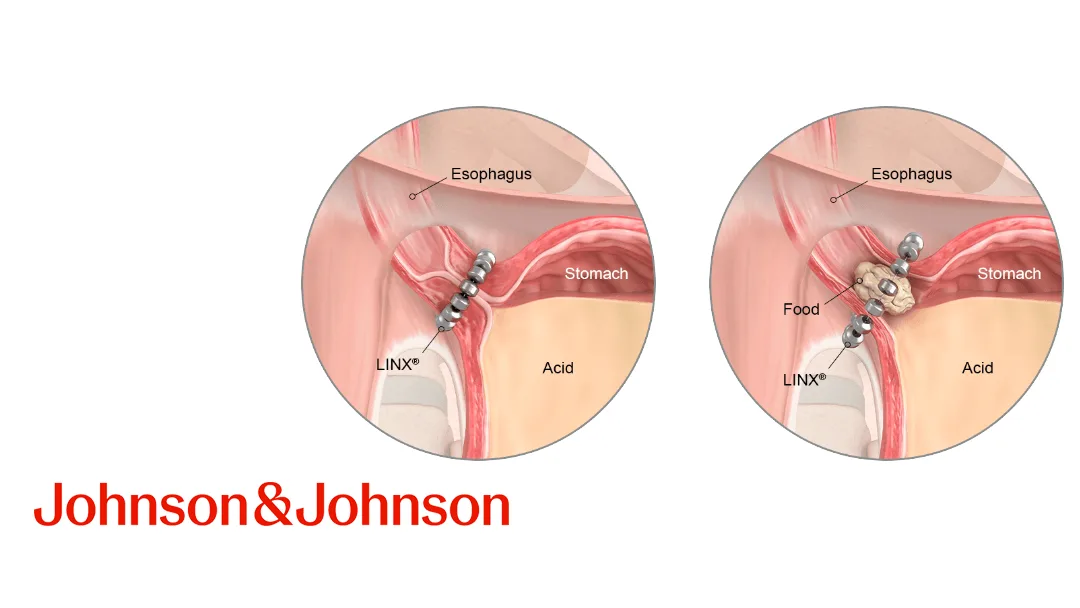
US giant Johnson & Johnson (J&J, NYSE: JNJ) disclosed that it will cease sales of its Linx Reflux Management System outside the United States from the end of March. The decision, described as a “commercial” choice, was communicated to physicians in a letter dated September 17, according to Bloomberg.
Impact on Treatment Landscape
- Surgeons warn of a decade‑long setback for patients with severe gastro‑oesophageal reflux disease (GERD), as the Linx system was one of the few minimally invasive options available worldwide.
- Lung‑transplant patients may also feel the ripple effect, since the Linx system had been used to reduce reflux‑related complications that can jeopardize transplant outcomes.
Company Position
- J&J emphasized that the withdrawal is unrelated to safety or efficacy concerns.
- The company cited “certain countries” as the target of the halt, underscoring a strategic realignment of its product portfolio in those markets.
Market Context
- The global GERD device market is projected to reach $4 billion by 2030; the Linx system represented a significant share of that value in regions outside the U.S.
- Competitors such as EndoSurgery and MucosalTech are poised to fill the vacuum, potentially accelerating the adoption of alternative technologies like endoscopic fundoplication.
What This Means for Stakeholders
- Patients in affected countries face limited access to a proven, implantable solution for refractory GERD.
- Physicians will need to explore alternative therapeutic pathways, including medical therapy escalation or other surgical devices.
- Investors may reassess the long‑term revenue potential of J&J’s gastro‑enterology portfolio, especially in emerging markets.-Fineline Info & Tech