BeOne Medicines Ltd. (NASDAQ: ONC; HKG: 6160; SHA: 688235) announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation (BTD) to its next‑generation BCL2 inhibitor, Sonrotoclax, for the treatment of adult patients with relapsed or refractory (R/R) mantle cell lymphoma (MCL). The designation represents the first BTD for Sonrotoclax and the second for BeOne’s hematology portfolio.
Key Highlights
| Item | Detail |
|---|---|
| Drug | Sonrotoclax (BCL2 inhibitor) |
| Indication | Adult R/R mantle cell lymphoma (MCL) |
| FDA Designations | Breakthrough Therapy, Fast‑Track, Orphan Drug (MCL, WM, MM, AML) |
| Pipeline Impact | Second BTD for BeOne, reinforcing leadership in hematology |
What the Designation Means
- Accelerated Development Pathway – Fast‑track review, potential priority status in clinical trials, and more frequent FDA interactions.
- Clinical Momentum – Builds on earlier Fast‑Track and Orphan Drug approvals for Sonrotoclax in MCL, Waldenström’s macroglobulinemia (WM), multiple myeloma (MM), and acute myeloid leukemia (AML).
- Strategic Advantage – Positions BeOne as a leading developer of targeted therapies for rare and high‑need hematologic malignancies.
About Sonrotoclax
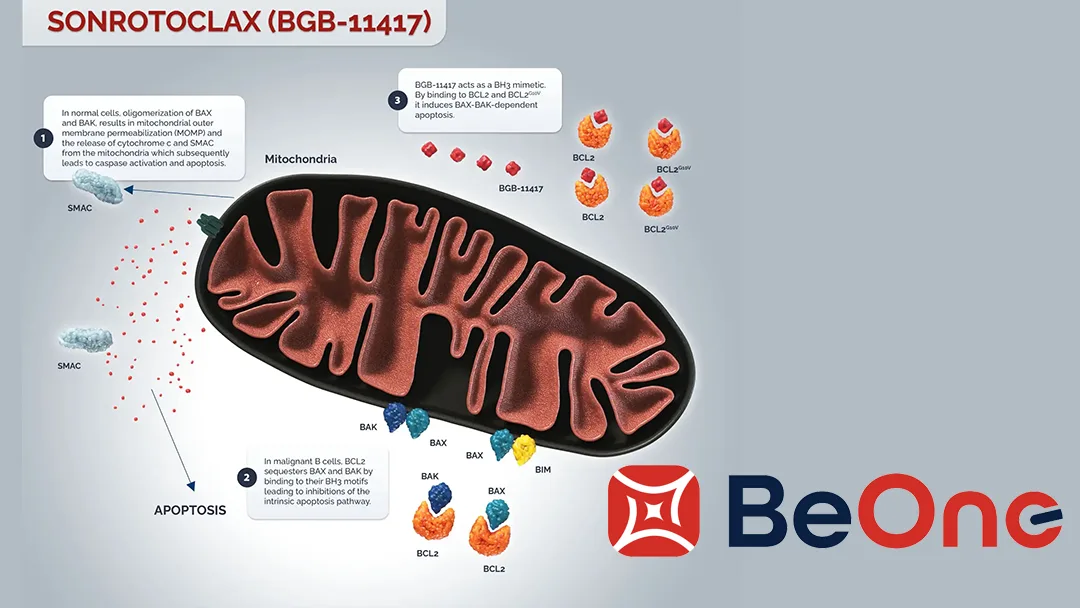
Sonrotoclax is a next‑generation, potentially best‑in‑class BCL2 inhibitor designed to induce apoptosis in malignant B‑cells. Preclinical and early‑phase data demonstrate potent activity against MCL cells, including those resistant to standard therapies.
About BeOne Medicines
BeOne Medicines is a global biotechnology company focused on discovering and developing innovative therapies for hematologic and solid‑tumor malignancies. The company’s portfolio spans multiple therapeutic classes, with a particular emphasis on targeted small molecules and biologics.
Forward‑Looking Statements
This release contains forward‑looking statements regarding the FDA designations, clinical development plans, and potential market impact. These statements are based on current expectations and are subject to risks and uncertainties, including regulatory approval, clinical outcomes, and market acceptance.-Fineline Info & Tech