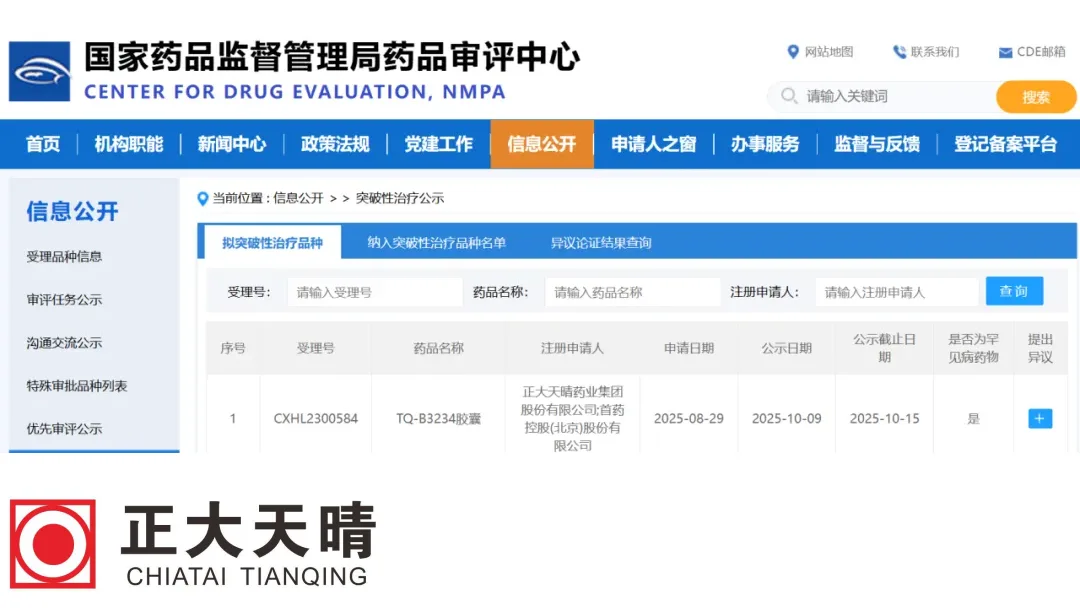
Jiangsu Chia Tai Tianqing Pharmaceutical Co., Ltd. (CTTQ), a subsidiary of China‑based Sino Biopharmaceutical Ltd. (HKG: 1177), today announced that its Class 1 innovative drug TQ‑B3234 Capsule—a selective MEK1/2 inhibitor co‑developed with Shouyao Holdings—has been granted Breakthrough Therapy Designation in China. The indication is the treatment of symptomatic, inoperable plexiform neurofibromas (PN) in adults with Neurofibromatosis Type 1 (NF1).
About TQ‑B3234
| Feature | Detail |
|---|---|
| Drug class | Selective MEK1/2 inhibitor |
| Target | RAS‑RAF‑MEK‑ERK signaling pathway |
| Indication | Symptomatic, inoperable PN in adult NF1 patients |
| Designation | Chinese Breakthrough Therapy (BT) status |
- Mechanism – Inhibits MEK1/2, upstream regulators of ERK, thereby suppressing tumor growth driven by RAS‑mutated pathways.
- Clinical promise – Offers a non‑surgical therapeutic option for a disease where surgery is often high‑risk and prone to recurrence.
Context: Neurofibromatosis Type 1
- Genetics – Autosomal dominant disorder caused by NF1 gene mutations; accounts for ~96 % of neurofibromatosis cases.
- Incidence – ~5 per million live births in China (2023 data).
- Rare‑Disease Status – Added to China’s “Second National List of Rare Diseases” in September 2023.
- Clinical burden – 30–60 % of NF1 patients develop plexiform neurofibromas, a leading cause of pain, disfigurement, and malignant transformation.
Significance of Breakthrough Therapy Designation
- Accelerated Development – Enables faster regulatory review and priority access to clinical trial data.
- Patient Impact – Potential to fill a critical unmet need for adults with inoperable PN.
- Strategic Advantage – Positions CTTQ and Sino Biopharmaceutical at the forefront of rare‑disease drug development in China.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech