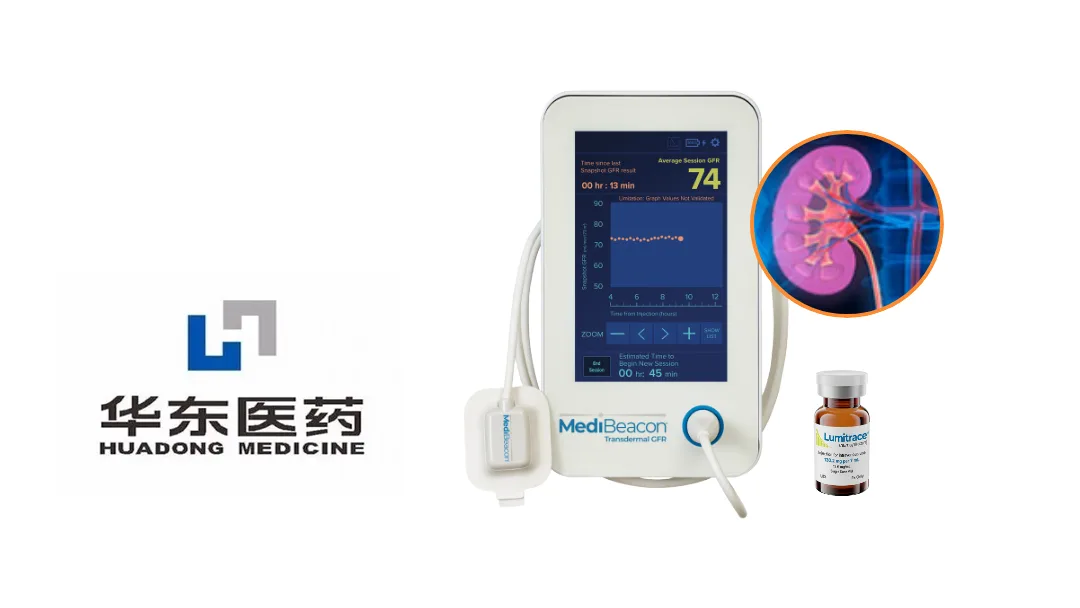
Huadong Medicine (SHE: 000963) announced that China’s National Medical Products Administration (NMPA) has granted marketing approval for Relmapirazin Injection, a Class I innovative drug. The injection is an exogenous fluorescent tracer that must be used in concert with the Transdermal Glomerular Filtration Rate (TGFR) Measurement Device from MediBeacon Inc. to accurately assess patients’ glomerular filtration rate (GFR).
Product Overview
| Component | Description |
|---|---|
| Relmapirazin Injection | Class I fluorescent tracer for GFR assessment |
| TGFR Device | First‑ever approved point‑of‑care transdermal GFR meter |
| Co‑development | Joint effort between Zhongmei Huadong (Huadong subsidiary) and MediBeacon Inc. (US partner) |
| Regulatory Status | NMPA marketing approval for drug (Relmapirazin) and device (TGFR) – device approval received Feb 2025 |
Strategic Implications for Huadong Medicine
- Integrated Diagnostic Ecosystem – The drug–device combination offers a streamlined, bedside solution for real‑time renal function monitoring, filling a critical gap in outpatient nephrology care.
- Exclusive Asian Rights – Huadong holds commercialization rights in mainland China, Hong Kong, Taiwan, Singapore, Malaysia, and 25 additional Asian markets, positioning the company to capture a large share of the growing renal diagnostics market.
- Regulatory Compliance – Separate registration pathways for the drug and device reflect China’s stringent regulatory framework, ensuring full compliance and market access without cross‑product conflicts.
Market Outlook
- Growing Demand – Chronic kidney disease prevalence is rising in Asia, driving demand for rapid, non‑invasive GFR testing.
- Competitive Advantage – No other product currently pairs a fluorescent tracer with a transdermal GFR meter, giving Huadong a first‑mover edge.
- Revenue Potential – The dual‑product strategy opens multiple revenue streams: drug sales, device sales, and associated consumables.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.-Fineline Info & Tech