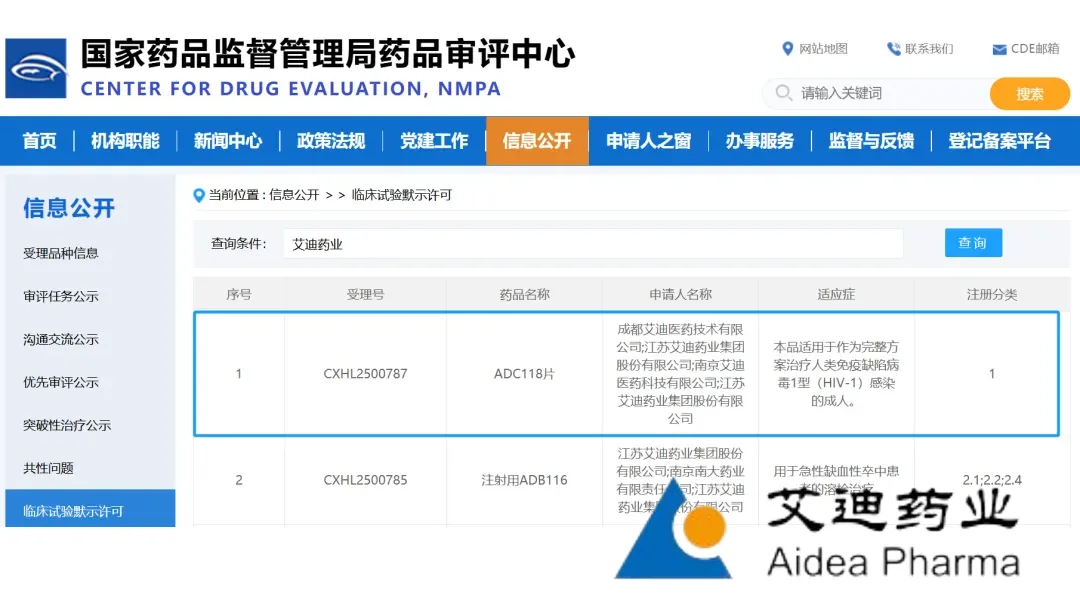
Jiangsu Aidea Pharmaceutical Co., Ltd. (SHA: 688488) announced today that its self‑developed Class 1 anti‑HIV drug, ADC118 Tablets, has received regulatory clearance to commence clinical trials in China. The triple fixed‑dose combination (ACC017/FTC/TAF) marks the first domestically‑developed HIV integrase‑inhibitor fixed‑dose combination (FDC) to enter the Chinese clinical stage.
Key Highlights
- Clinical‑Trial Approval – ADC118 is cleared to begin Phase I/II testing in China for the treatment of adult HIV‑1 infection.
- Product Profile – The FDC contains:
- ACC017 (Asuptegravir, ASU) – a novel Class 1 integrase inhibitor developed by Aidea.
- Emtricitabine (FTC) – a nucleoside reverse‑transcriptase inhibitor.
- Tenofovir Alafenamide (TAF) – a nucleotide reverse‑transcriptase inhibitor.
- Mechanism of Action – ASU inhibits HIV‑integrase, blocking integration of the viral genome into host DNA.
- Development Status – ASU has already entered Phase III trials worldwide and will be evaluated in combination with FTC/TAF in China.
Strategic Significance
- Domestic Innovation Milestone – ADC118 is the first Chinese‑origin HIV integrase‑inhibitor FDC, positioning Aidea as a leader in antiretroviral innovation.
- Regulatory Milestone – The approval reflects the Chinese authorities’ commitment to expanding access to cutting‑edge HIV therapies.
- Pipeline Expansion – Successful clinical data could fast‑track ADC118 to global markets and create a new revenue stream for Aidea.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties. Actual results may differ materially.