Lepu (Beijing) Medical Technology Co., Ltd. (SHE: 300003) announced that its wholly‑owned subsidiary Shanghai Minwei Biotechnology Co., Ltd. has entered into an exclusive licensing agreement with Sidera Bio Aps. (Denmark) for the triple‑receptor agonist MWN105 injection.
Deal Overview
| Item | Details |
|---|---|
| Licensee | Sidera Bio (Denmark) – exclusive global rights outside Greater China (Mainland China, Hong Kong SAR, Macau SAR, Taiwan) |
| Up‑front & Near‑Term Payments | $35 million (initial investment + early milestones) |
| Cumulative Milestones | Up to $1.01 billion tied to clinical‑development milestones and commercial‑sales thresholds |
| Equity Component | Minwei to acquire a 9.99 % stake in Sidera Bio |
| Royalty Structure | Tiered royalties on net annual sales in licensed territories |
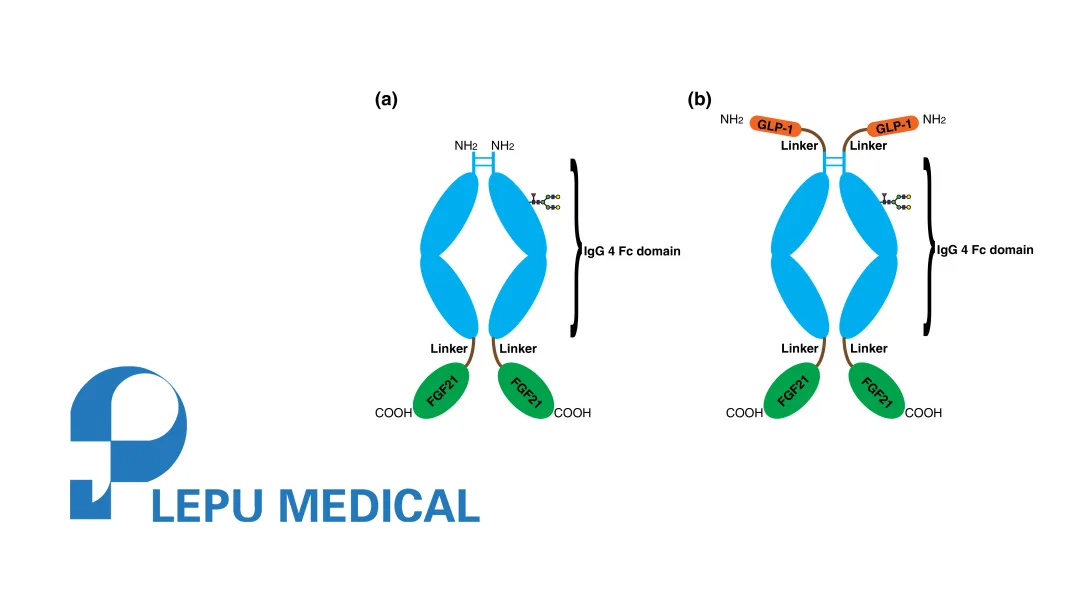
| Product | MWN105 injection – GLP‑1/GIP/FGF21 triple receptor agonist for diabetes, obesity & metabolic disease |
| Regulatory Status | Approved in Mainland China; ongoing Phase II/III trials in China |
Strategic Rationale
- Global Reach – Sidera Bio gains a platform‑class asset with a clear path to markets in Europe, the U.S., and emerging economies, while Lepu/Minwei retains rights in the high‑growth Greater China region.
- Financial Upside – The $35 M upfront and $1.01 B potential milestone structure provide significant upside for Minwei and, by extension, Lepu shareholders.
- Portfolio Synergy – MWN105’s triple‑agonist mechanism complements Sidera’s existing pipeline in metabolic disorders, accelerating time‑to‑market for a differentiated therapy.
- Equity Alignment – The 9.99 % equity stake aligns long‑term interests of both parties and offers Minwei exposure to Sidera’s future valuation.
About MWN105
- Mechanism: Simultaneous activation of GLP‑1, GIP, and FGF21 receptors, targeting glucose regulation, appetite control, and lipid metabolism.
- Indications: Type 2 diabetes, obesity, and broader metabolic disease spectrum.
- Clinical Progress: Completed Phase II in China with positive efficacy and safety signals; Phase III enrollment underway.
About the Companies
- Lepu (Beijing) Medical Technology Co., Ltd. – A leading Chinese integrated medical‑device and pharmaceutical group, listed on the Shenzhen Stock Exchange (SHE: 300003).
- Shanghai Minwei Biotechnology Co., Ltd. – Lepu’s biotech arm focused on innovative peptide‑based therapeutics.
- Sidera Bio A/S – Denmark‑based biopharma specializing in metabolic‑disease drug development, with a robust global commercialization platform.
Forward‑Looking Statements
This release contains forward‑looking statements that involve risks and uncertainties, including assumptions about regulatory approvals, clinical trial outcomes, and market acceptance. Actual results may differ materially.-Fineline Info & Tech