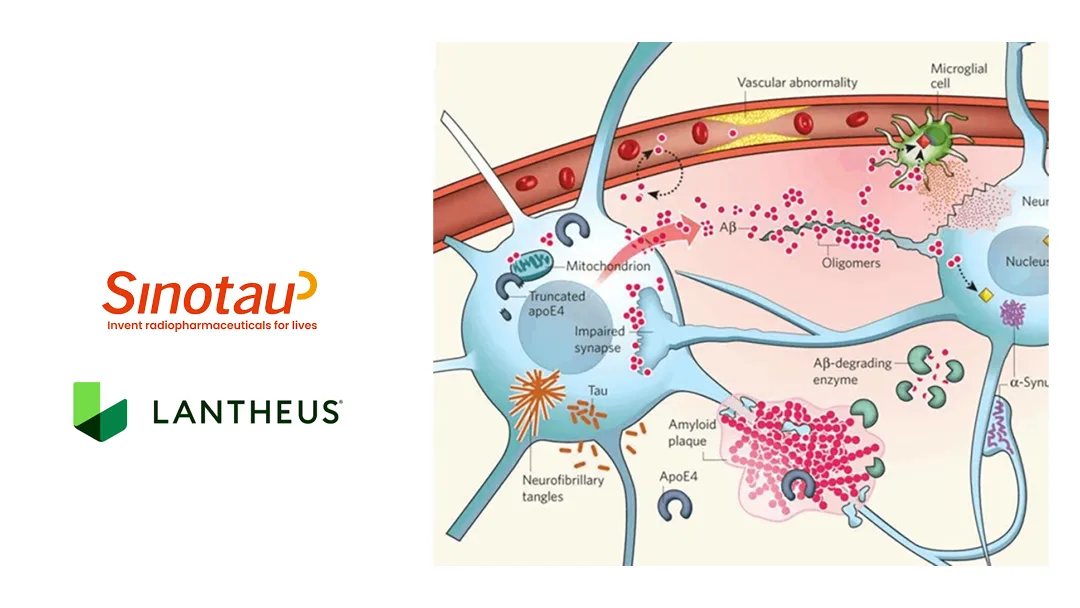
Beijing Sinotau International Pharmaceutical Technology Co., Ltd. announced today that the New Drug Application (NDA) for MK‑6240/XTR006, developed by its partner Lantheus Holdings, Inc. (NASDAQ: LNTH), has been accepted by the U.S. Food and Drug Administration (FDA). The agent is designed to detect tau neuro‑fibrillary tangle (NFT) pathology in patients with cognitive impairment who are being evaluated for Alzheimer’s disease.
FDA Acceptance & Timeline
- NDA Status – Full acceptance by the FDA, following prior Fast‑Track designation.
- PDUFA Action Date – August 13, 2026, giving a clear regulatory runway.
- Manufacturing Rights – Sinotau holds exclusive rights for development, production, and commercialization in China since 2017.
Clinical Evidence
- Phase 3 Trials – Two pivotal studies in the U.S. confirmed high sensitivity and specificity for detecting tau NFTs in early Alzheimer’s disease.
- Co‑Primary Endpoints – Both sensitivity and specificity achieved the required thresholds, supporting diagnostic accuracy.
- China Phase 3 – The agent is also active in a domestic Phase 3 program, underscoring Sinotau’s global‑local strategy.
Strategic Implications
- Diagnostic Gap Fulfillment – MK‑6240 offers a unique tau‑targeted PET imaging platform, addressing the unmet need for early Alzheimer’s biomarkers.
- Market Lead – A first‑in‑class product positions Sinotau and Lantheus as pioneers in neuroimaging diagnostics.
- Commercial Opportunity – With exclusive Chinese rights and an FDA‑approved NDA, Sinotau can rapidly scale production and distribution once the PDUFA milestone is met.
Bottom Line
Sinotau’s FDA NDA acceptance for MK‑6240/XTR006 marks a pivotal milestone for Alzheimer’s diagnostics. The agent’s proven clinical performance, combined with Sinotau’s exclusive Chinese manufacturing and commercialization authority, positions the company—and its Lantheus partner—to capture a substantial share of the rapidly expanding dementia imaging market.-Fineline Info & Tech