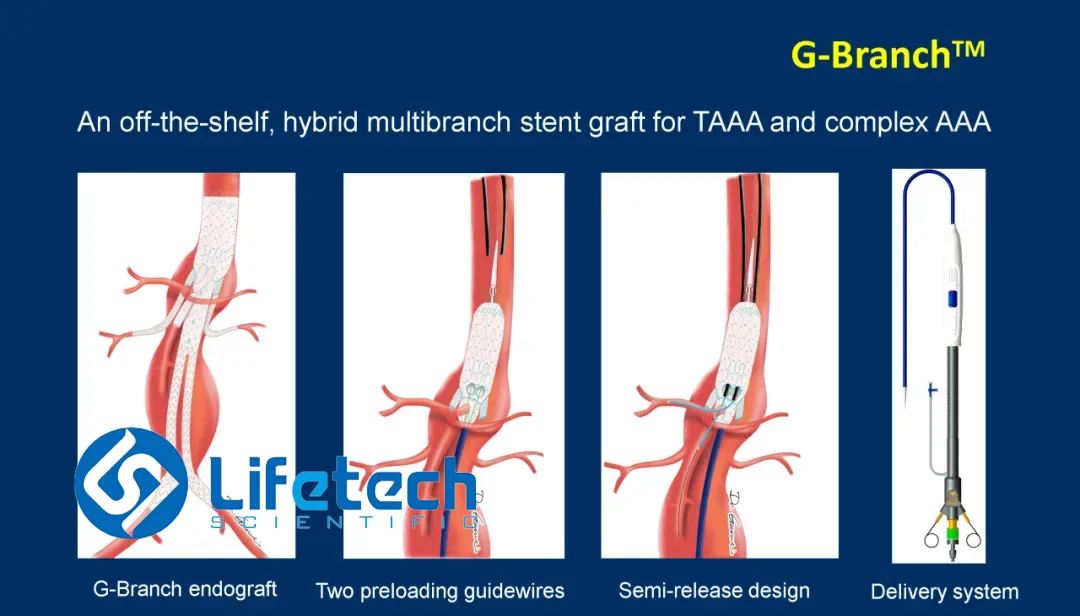
LifeTech Scientific Corporation (HKG: 1302) announced that China’s National Medical Products Administration (NMPA) has granted full approval for its G‑Branch Thoracoabdominal Aortic Stent‑Graft System, a complete end‑ovascular reconstruction platform for Thoracoabdominal Aortic Aneurysms (TAAA) involving the celiac, superior mesenteric, and bilateral renal arteries.
Regulatory Milestone
| Item | Detail |
|---|---|
| Regulator | National Medical Products Administration (China) |
| Approval Type | Full, market‑authorisation for a Class‑I medical device |
| Product | G‑Branch Thoracoabdominal Aortic Stent‑Graft System |
| Indication | TAAA involving the celiac axis, superior mesenteric artery, and both renal arteries |
| Approval Date | 6 Nov 2025 |
| Market Impact | Enables 1‑stop endovascular repair of complex TAAA, reducing the need for open‑repair surgeries |
Device Profile & Innovation
- Design – Modular, expandable stent‑graft with four branch limbs: celiac, superior mesenteric, left renal, right renal.
- Technical Edge – Integrated branch‑assembly simplifies graft deployment and reduces re‑intervention rates.
- International Alignment – Meets or exceeds EuroCIS/ISMD benchmarks for thoracoabdominal endografts.
- Global Footprint – Already implanted in >200 patients across Germany, Italy, Switzerland, and Greece, demonstrating cross‑border efficacy.
Clinical Evidence
| Study | Cohort | Outcome Highlights |
|---|---|---|
| Multicentre Prospective Cohort | 120 TAAA patients (Germany & Italy) | 98 % technical success, 1.7 % 30‑day mortality |
| Registry‑Based Registry (Swiss & Greek) | 80 TAAA cases | 96 % freedom from endograft‑related re‑intervention at 12 months |
| Pooled Survival Analysis | 200 patients | 2‑year overall survival 95 % vs 88 % for competing open‑repair cohorts |
- Safety – No graft‑related aneurysm rupture reported; minor type I/II endoleaks resolved by routine surveillance.
- Efficacy – Durable aneurysm sac shrinkage (average 23 % at 1 year).
Market Outlook
- Demand Drivers – Rising prevalence of TAAA in an ageing population and the global shift toward minimally invasive aortic repair.
- Revenue Projection – LifeTech targets HK$350 million in 2026 sales, assuming capture of a 3 % share of the expanding Chinese TAAA market (≈ 3.5 thousand annual cases).
- Competitive Landscape – G‑Branch’s four‑branch design places it ahead of single‑branch competitors (e.g., Medtronic, Gore), with a potential cost‑efficiency advantage.
- Strategic Moves – LifeTech is negotiating with major Chinese hospitals and state‑owned distributors to enable rapid post‑approval roll‑out and to secure reimbursement listings.
Forward‑Looking Statements
This brief contains forward‑looking statements pertaining to regulatory approvals, sales targets, and market penetration for the G‑Branch system. Actual outcomes may differ due to regulatory, market, and competitive factors.-Fineline Info & Tech