Nantong DeliNova Therapeutics Co., Ltd. announced today the successful closing of a seed‑stage financing round that raised almost RMB 60 million.
Funding Partners
| Investor | Affiliation |
|---|---|
| Hanyuan Asset (Shanghai Jiao Tong University Foundation) | University‑linked venture |
| Legend Capital | Private equity |
| CAS Star | Science‑centric venture |
| Vision Investment | Venture capital |
| Hongfeng Venture | Early‑stage investment fund |
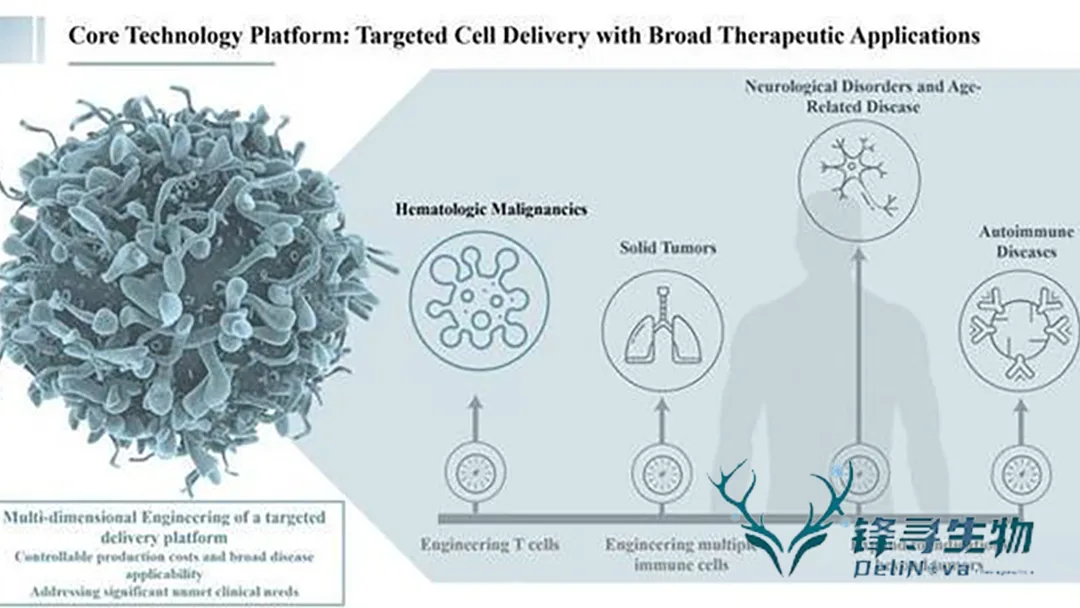
The capital injection will accelerate the development of DeliNova’s proprietary in‑vivo CAR‑T technology platform, expand its team, and support the clinical translation of its first candidate product targeting hematological malignancies and autoimmune diseases.
DeliNova’s Business Model
- Core Focus – Development of lenti‑viral vectors that deliver CAR genes directly to T cells in vivo, obviating the need for ex vivo cell manipulation.
- Strategic Advantage – Targeted, efficient transduction promises higher CAR‑T cell specificity and reduced manufacturing complexity.
- Pipeline – Clinically validated targets underpin a first‑in‑class platform, with investigator‑initiated trials (IITs) slated for Q4 2025 in hematologic cancers and IND preparation in progress.
Clinical Milestones
| Phase | Timeline | Focus |
|---|---|---|
| IIT (Hematologic Malignancies) | Q4 2025 | First in‑vivo CAR‑T study |
| IND Preparation | Q4 2025 | Regulatory submission groundwork |
| IIT (Autoimmune & Solid Tumors) | 2026 | Expansion to broader disease indications |
Forward‑Looking Statements
This release contains forward‑looking statements regarding future funding utilization, clinical trial initiation, and regulatory approval prospects. Actual results may differ materially.-Fineline Info & Tech