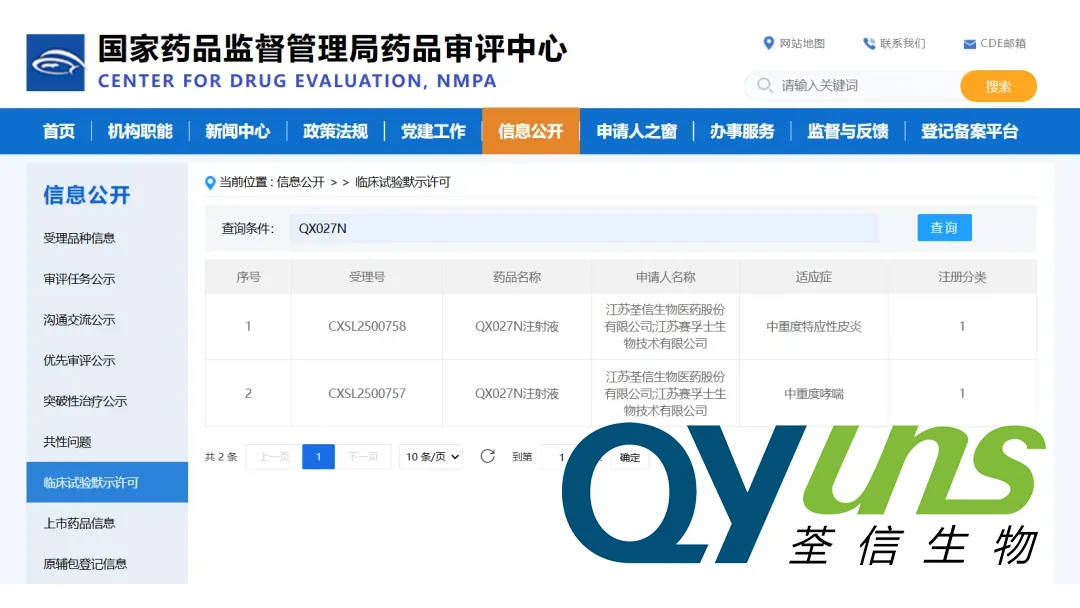
Qyuns Therapeutics Co., Ltd. (HKG: 2509) announced that China’s National Medical Products Administration (NMPA) has granted two clinical‑trial approvals (Acceptance Nos.: CXSL2500757 & CXSL2500758) for its first‑in‑class, long‑acting bispecific antibody injection QX027N. The drug is slated for the treatment of asthma and atopic dermatitis.
Regulatory Highlights
| Item | Detail |
|---|---|
| Company | Qyuns Therapeutics Co., Ltd. (HKG: 2509) |
| Agency | National Medical Products Administration (NMPA) |
| Approval Type | Clinical‑trial authorization (Phase I/II) |
| Acceptance Nos. | CXSL2500757, CXSL2500758 |
| Therapeutic Indications | Asthma & Atopic Dermatitis (autoimmune‑allergy) |
| Date Granted | 14 Nov 2025 |
QX027N – Product Snapshot
- Format: Long‑acting bispecific antibody (humanized IgG1‑based).
- Mechanism: Simultaneous engagement of IL‑13 and IL‑4Rα to block key Th2‑driven pathways in airway and skin inflammation.
- Pre‑clinical Data: Superior suppression of eosinophilic inflammation in rodent asthma models and durable skin barrier healing in atopic dermatitis models.
- Development Pathway: Expected to progress to Phase IIA in late 2026, contingent upon safety and PK data.
Company Profile & Recent Funding
- Founded: 2015; focus on biological therapies for autoimmune and allergic disorders.
- Capital Raise: August 2025 – 5 million shares sold to TruMed Health Innovation Fund LP, raising HKD 100 million (≈ US$13 million).
- Use of Proceeds: R&D for QX027N and expansion of the broader immunology pipeline, including a next‑generation IL‑31 blocker.
- Strategy: Leverage the bispecific platform to gain advantages in efficacy and dosing convenience, aiming for a first‑in‑class market entrance.
Market Implications
- Asthma & Atopic Dermatitis Landscape: 35 million asthmatics and 20 million atopic dermatitis patients globally; short‑acting biologics dominate, leaving a gap for long‑acting, dual‑target agents.
- Competitive Edge: QX027N’s bispecific design may reduce frequent dosing and improve patient adherence compared with existing monoclonal antibodies.
- Potential Revenue: If approved, QX027N could generate HK$5–8 billion (USD $650–$1 billion) in 2028‑2030, assuming a 1–2 % market share in Hong Kong and 0.5 % in China.
Forward‑Looking Statements
This brief includes forward‑looking statements regarding regulatory approvals, clinical development, funding impact and commercial prospects. Results may differ due to market, regulatory, and operational risks.-Fineline Info & Tech