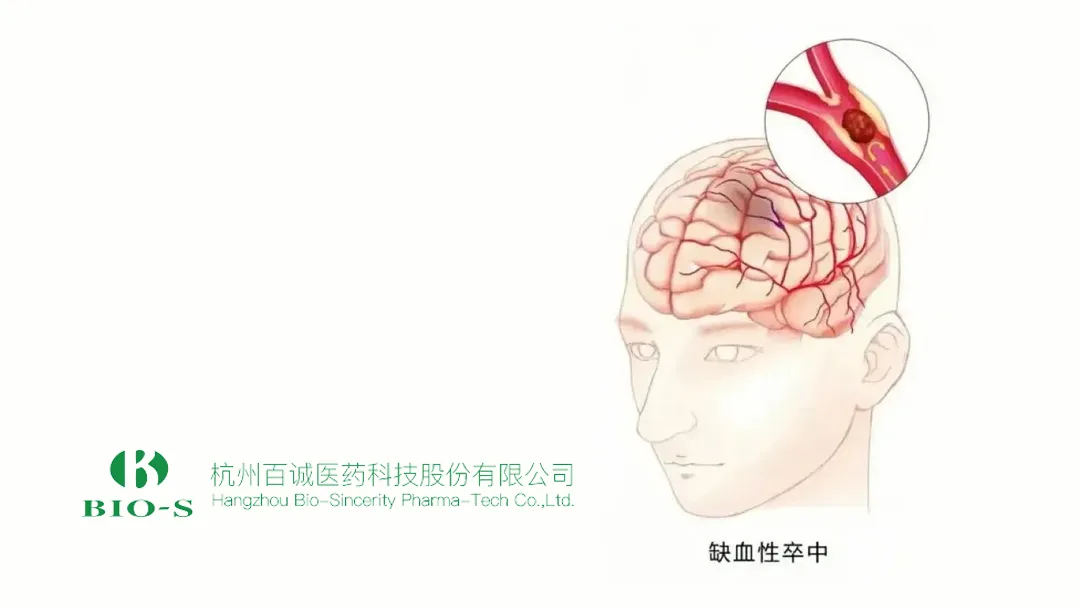
Hangzhou Bio‑Sincerity Pharma‑Tech Corp., Ltd. (SHE: 301096) announced that its BIOS2242 oral solution has received clinical‑trial approval from the China National Medical Products Administration (NMPA) for the treatment of mild to moderate acute ischemic stroke. The drug is classified as a Category 2.2 modified product, designed specifically for patients who cannot safely swallow solid oral dosage forms.
Regulatory Milestone
| Item | Detail |
|---|---|
| Company | Hangzhou Bio‑Sincerity Pharma‑Tech Corp., Ltd. (SHE: 301096) |
| Product | BIOS2242 oral solution (Category 2.2 modified drug) |
| Indication | Mild‑to‑moderate acute ischemic stroke |
| Regulatory Body | China NMPA |
| Approval Type | Clinical‑trial (Phase I/II) authorization – 17 Nov 2025 |
| Target Population | Patients with cerebral ischemia who experience dysphagia, coma, impaired consciousness, paralysis or limb numbness |
Why an Oral Solution Matters
- Dysphagia‑Friendly – Stroke patients often lose the ability to swallow tablets or capsules. An aqueous solution eliminates the risk of aspiration and ensures accurate dosing.
- Rapid Onset – Liquid formulations can be absorbed more quickly than solid forms, a critical advantage in the narrow therapeutic window of acute ischemic stroke.
- Simplified Administration – Caregivers can deliver the medication via syringe or feeding tube without the need for tablet crushing or compounding.
Market & Clinical Impact
- Unmet Need – In China, > 2 million new ischemic‑stroke cases are reported annually; roughly 30 % present with swallowing difficulties that limit conventional oral therapy.
- Revenue Potential – Assuming a 10 % market capture of the dysphagic stroke segment (≈ 600,000 patients) at an average price of CNY 12,000 per treatment course, BIOS2242 could generate ≈ CNY 7.2 billion (~US$1.0 billion) in annual sales once fully commercialized.
- Strategic Fit – The approval strengthens Hangzhou Bio‑Sincerity’s pipeline in neuro‑vascular therapeutics and positions the company for future Phase III expansion and potential global partnership discussions.
Forward‑Looking Statements
This brief contains forward‑looking statements regarding regulatory timelines, market size, and revenue expectations for BIOS2242. Actual results may differ due to clinical outcomes, additional regulatory requirements, and competitive dynamics.-Fineline Info & Tech